Figure 8.
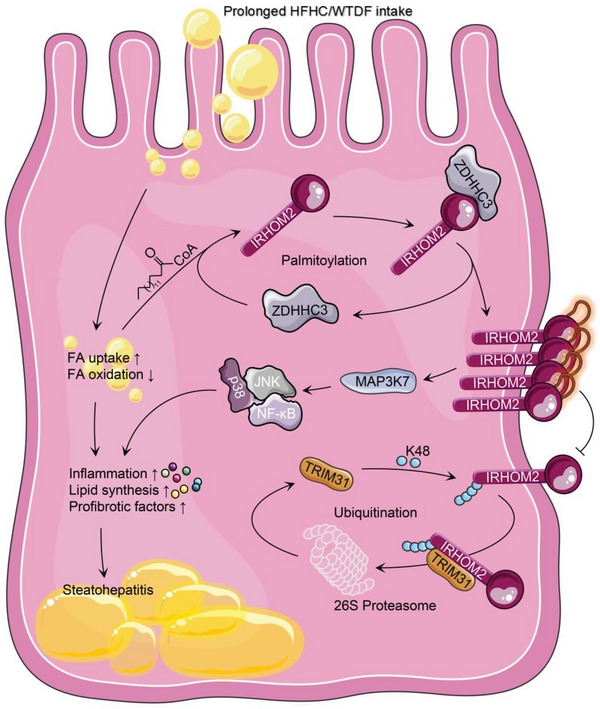
Palmitoylation maintains IRHOM2 by intercepting its ubiquitination and proteasome degradation. Working model of the regulation of palmitoylated IRHOM2 by palmitoyltransferase ZDHHC3 retards ubiquitin‐proteasome degradation of IRHOM2. Prolonged HFHC or WTDF intake dramatically increases circulating free fatty acid levels and palmitate (C16:0) to promote formation of palmitoylated IRHOM2. Elevated palmitoylation of IRHOM2 by ZDHHC3 facilitates its membrane localization and accumulation, and stabilization. Excessive IRHOM2 abundance significantly recruits its downstream signaling MAP3K7‐JNK‐NF‐κB p65 axis hyperactivation, thereby accelerating steatohepatitis progression in response to high‐energy diet challenge. In the meantime, palmitoylation‐stabilized IRHOM2 positively blocks its ubiquitination and TRIM31‐mediated proteasome degradation via decreasing ubiquitin of K48 linkage. Targeting palmitoylation using a pharmacological inhibitor (2‐BP) provides a potential therapeutic strategy to regain IRHOM2 ubiquitination and proteasome degradation.
