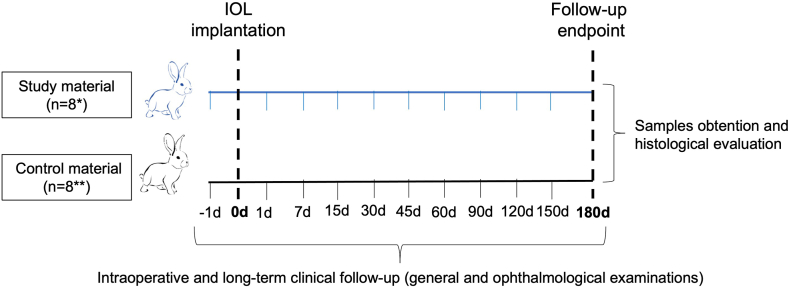
Fig. 4.
Study design and timeline. This experiment followed UNE-EN ISO 11979-5:2020 Ophthalmic implants. Intraocular lens and ISO 10993-6:2016 Biological evaluation of medical devices. Animals were randomly divided into two groups (n = 8 each) according to the implant materials (Study: Accommodative LUZ IOL; or Control: EyeCee® IOL). During the implantation procedure, intraoperative follow-up was performed. Long-term clinical follow-up consisted of examining the animal's general condition and eyes on the day before surgery and periodically until the end of the study at 180 days. Finally, the eyes were enucleated and evaluated macro/microscopically. d: days; IOL: intraocular lens. *7 animals completed the follow-up. **5 animals completed the follow-up.