Abstract
Aim
This study aimed to evaluate the biocompatibility, flexural strength, and surface roughness of polymethyl methacrylate (PMMA) containing Copper Oxide Nanoparticles (CuO NPs) at different concentrations.
Methods
25 heat-polymerized PMMA wax patterns fabricated in 5 groups containing 0.5, 5, 50, and 500 μg/ml CuO NPs and nanoparticle (NP)-free PMMA discs were prepared. 5 growth mediums (DMEM with 10% FBS and 1% penicillin-streptomycin) without disks were also incubated similarly to serve as the control groups. The cytotoxicity of the discs was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay on cultured Human Gingival Fibroblasts. The number of 1.3 × 104 cells were seeded in each well of 96-well plates (5 wells for the extract of each specimen). Days 1, 3, 5, and 7 were the intervals that the culture media were in direct contact with the PMMA discs for either 24 or 72 h. After that, a total of 40 specimens with 65 × 10 × 2.5 mm dimensions were prepared in five groups (n = 8). The specimens were subjected to a rugosimeter for the evaluation of surface roughness. The flexural strength test was performed using a universal testing machine. Microscopic evaluation was performed for the dispersion of the NPs. Non-parametric Kruskal-Wallis test and parametric one-way ANOVA test were used for data analysis.
Results
The samples containing 500 μg/ml NPs showed the lowest percentage of cell viability at all incubation periods, while the highest cell viability was observed in NP-free PMMA 24 h after the seventh day of incubation. NPs at 50 and 500 μg/ml concentrations showed strongly significant differences in cytotoxicity compared to the 0 concentration and the control group (p < 0.001). Although all the samples demonstrated an increasing pattern of cell viability on the third, fifth, and seventh days, the percentage of cell viability was significantly lower after 72 h than after 24 h in all incubation periods (p < 0.001). NPs significantly increased flexural strength (p = 0.005) but did not affect the surface roughness of the PMMA discs (p = 0.396).
Significance
The CuO NPs were cytotoxic only when applied in high concentrations, but presented a descending trend over time. No cytotoxic effect was observed in the experimental groups after seven days of incubation. Furthermore, CuO NPs increased flexural strength, but the surface roughness of the PMMA discs was not affected.
Keywords: Materials testing, Flexural strength, Cupric oxide, Nanoparticle, Acrylic resins
Highlights
-
•
Polymethyl methacrylate (PMMA) containing low-dose copper oxide nanoparticles (CuO NPs) has no toxic effects.
-
•
CuO NPs increase the flexural strength of the denture base resin (PMMA).
-
•
CuO NPs do not affect the surface roughness of the denture base resin (PMMA).
-
•
Low-dose CuO NPs have favorable effects when incorporated into denture base resins.
1. Introduction
Polymethyl methacrylate (PMMA) is mostly used in dentistry for different purposes such as denture base materials, obturators, and functional appliances due to its favorable properties [1]. However, it has some relative inadequacies and poor mechanical properties, which can lead to denture fracture [2,3]. The major issue regarding acrylic appliances is microbial plaque accumulation, since the porous and non-smooth surface can act as an ideal incubator for microorganisms, especially fungal infections [4,5]. The most important adverse effect of antifungal treatment is medication resistance [6]. Hence, numerous studies have been devoted to developing new denture base resin containing antimicrobial agents, mostly nano-sized particles [[7], [8], [9], [10]]. Moreover, although PMMA meets the required properties for denture base resin, some of its mechanical properties such as flexural strength, fatigue failure, and thermal conductivity, are not optimal and need further improvement [2,11].
Nanotechnology is a new promising field with multiple potential applications in medicine and dentistry. In this field, various Nanoparticles (NPs) have been added to PMMA to enhance its mechanical and physical properties and to make use of its antimicrobial effects [4,[12], [13], [14]]. Nanomaterials such as carbon-based hydroxyapatite, silica, TiO2, Ag2O, ZnO, and ZrO2 revealed cytotoxicity to Candida and other microbes present in oral biofilm present on PMMA denture surfaces. Nano toxicity may attribute to the direct interaction of nanoparticles with cell membranes, hindrance in protein synthesis and early adhesion, and therefore interfere with the physiology of pathogens. But only some metal oxide NPs such as Copper Oxide (CuO) NPs which are ionic have antimicrobial properties since they have a high surface area and different crystalline structures with more reactive sites [4]. Other adverse effects such as change of esthetics, microspores, roughening, and/or pit formations, adhesive fractures, and high cost of material are listed in literature for other metal oxide NPs [15].
Copper Oxide (CuO) NPs have been extensively used in industrial (e.g., batteries, solar cells, heat transfer materials, and gas sensors) and biomedical fields due to their multifunctional properties [16,17]. Biomedical utilizations of CuO NPs have also been reported including drug delivery, virus detection, and antimicrobial activity. Considering their abundant resources and low cost, CuO NPs are promising antimicrobial elements for use in dentistry. The use of CuO NPs, as an antimicrobial agent, in dentistry has also been formerly claimed. For instance, CuO-coated brackets were found to reduce the colony formation of bacteria. In addition, CuO NPs significantly reduced the oral bacterial load, eventually inhibiting the growth of oral biofilm colonizers [[18], [19], [20], [21]].
Considering the abundant resources of Cu and its low cost in comparison to other particles such as Ag, CuO NPs can be used for their antibacterial, antifungal, and mechanical properties in prosthetic dentistry. Recent researchers revealed the antimicrobial effect of PMMA containing CuO NPs [[22], [23], [24]]. A recent study showed that adding CuFe2O4/Cu2O/CuO nanoparticles in the amount of 5 wt% can significantly enhance the antimicrobial effect of the resin [24].
However, the use of excessive CuO NPs is limited by their safety and toxicity [[24], [25], [26]]. The toxicity of NPs depends on different factors such as particle size, shape, concentration, coatings, cell type, and exposure condition and duration, which result in different situations that cannot be easily compared to each other [[22], [23], [24], [25], [26], [27], [28]]. Moreover, NPs may penetrate through biological barriers due to their small sizes and reach the internal parts of the body, provoking hazardous cellular damage. Despite the beneficial antimicrobial effects of CuO NPs, their cytotoxicity can adversely affect human cells. In the case of oral exposure to NPs, many internal organs may be exposed [29,30]. A previous investigation reported the higher toxic properties of CuO NPs compared to other metal NPs due to oxidative stress, especially on lung and laryngeal epithelial cells. The authors state that: “Our short-term exposure study of high-level induction of genotoxic response of CuO NPs will need to be further investigated to determine whether long-term exposure consequences may exist for CuO NPs application” [31].
Although evidence exists regarding the cytotoxicity of CuO NPs, it is still unclear how they act when incorporated in PMMA [[30], [31], [32], [33]]. Moreover, there is a paucity of data concerning its effects on mechanical properties such as flexural strength and surface roughness. As acrylic dentures are prone to fracture due to flexural fatigue, acryl's high flexural strength is vital for dentures' success [3]. To date, there is a lack of consensus regarding the optimized concentration of CuO NPs incorporated in PMMA, showing effective antimicrobial and mechanical properties without exerting cytotoxicity effects. Besides, no study has been done on the cytocompatibility of CuO-containing PMMA. Thus, the present study aimed to evaluate the cytotoxicity and mechanical properties of PMMA containing CuO NPs. The first null hypothesis was that PMMA containing CuO NPs is not cytotoxic to Human Gingival Fibroblast (HGF) cells. The second hypothesis was that CuO NPs do not affect flexural strength. And Finally, the third null hypothesis was that CuO NPs do not affect the surface roughness of the PMMA.
2. Materials and methods
2.1. PMMA-copper oxide nanoparticle samples fabrication
Heat-cure acrylic resin specimens were fabricated by investing disk-shaped wax patterns in stone molds within a dental flask. 25 wax patterns (10 × 2 mm) were invested in a dental stone (Fujirock EP; GC, Leuven, Belgium). After the setting of the stone, the flasks (61B Two Flask Compress; Handler Manufacturing, Westfield, NJ, USA) containing the wax patterns were opened and dewaxed in boiling water for 5 min. The acrylic resin specimens were fabricated in five groups. A group containing specimens composed of acrylic resin without NPs and four experimental groups containing specimens composed of acrylic resin with four different concentrations of CuO NPs (500, 50, 5, and 0.5 μg/ml) were considered. These concentrations were chosen according to previous studies which evaluated the antimicrobial effects of this nanoparticle incorporated in denture base resins [22,23]. The sample size was calculated using the sample means presented in a similar study [34]. For this, the MedCalc statistical software version 20.013 (Mariakerke, Belgium) with 90% power and α = 0.05 were used. The sample size was calculated to be at least n = 3 in each group. Therefore, a total of 25 specimens and 5 controls were fabricated. The five samples in each group were fabricated independently (n = 5).
For this purpose, the appropriate amount of CuO NPs (average size = 40 nm, 99.9% purity, Fanavaran Daneshgah, Isfahan, Iran) was added to Poly Methyl Methacrylate monomer (PMMA; SR Triplex Hot, Ivoclar Vivadent, Liechtenstein, Germany). The resultant suspension was then stirred by an ultrasonic homogenizer (BioLogics, Inc., Manassas, Virginia 20,109, USA) twice for 5 min at 20 kHz and 105 W to disperse NPs in the PMMA monomer and was mixed with PMMA powder at liquid: powder ratio of 1:3. When the PMMA-CuO NPs mixture reached the dough phase at room temperature, it was packed into flasks. The flask halves were closed and compressed using a hydraulic press under a 1250 kgf load for 5 min. Then, the flasks were placed in a water bath curing unit and were processed by heating to 74 °C for 90 min and to 100 °C for 30 min following the manufacturer's instructions. Once the polymerization process was finished, all the flasks were left to cool down in the water before de-flasking. After that, the flasks were opened and the specimens were removed from the flasks, finished, and polished using 220-2400-grit SiC sandpaper to obtain a highly polished surface, which is required for the denture resin outer surface to inhibit bacterial and fungal attachment and discoloration.
2.2. Cells and cell culture condition
Human gingival fibroblasts (HGFs) were used to determine the cytotoxic effects of PMMA-containing CuO NPs. The HGF 1- PI 1, C 165 cell line was derived from an explant culture of gingival biopsy taken from a normal 28-year-old Caucasian female. It was purchased from the Pasteur Institute of Iran in a culture medium containing 45 ml Dulbecco's Modified Eagle's Medium (DMEM), 10% Fetal Bovine Serum (FBS) and 1% penicillin-streptomycin. When the cells were 75–80% confluent, the passage was done.
2.2.1. Passaging
-
1.
The Phosphate Buffered Saline (PBS or balanced salt solution that is a Ca+2 and Mg+2-free solution) and growth media were pre-warmed to 37 °C in a water bath and the trypsin solution was thawed.
-
2.
The adherent cells were gently rinsed with PBS (in order to rinse FBS from the culture surface as it deactivates trypsin).
-
3.
The resulting solution was removed from the flask.
-
4.
1–2 ml of thawed trypsin solution was added to the flask and the contents were gently swirled to cover the cell layer.
-
5.
The vessel was incubated at 37 °C for 2–3 min as the next step.
-
6.
The cells were observed under a microscope. The detached cells appeared rounded and refractile.
-
7.
Once cells appeared detached, 2–3 vol of pre-warmed complete growth media were added to inactivate trypsin.
-
8.
The medium was gently dispersed by pipetting over the cell layer surface several times to ensure recovery of >95% of cells.
-
9.
The cell suspension was transferred to the tube and was gently centrifuged at 1500 rpm for 5 min.
-
10.
After removing the supernatant, the cell pellet was gently re-suspended in a pre-warmed complete growth medium by gentle pipetting.
-
11.
The cells were counted (as it is explained further) and diluted to the appropriate concentration for seeding.
-
12.
The appropriate volume of cell suspension was added to a new flask containing medium. (T-25 flasks have the capacity for 0.5–1 × 106 cells and T-75 flasks have the capacity for 1–2 × 106 cells.)
-
13.
The flask was placed in an incubator at 37 °C, 5% CO2, and 95% humidity.
2.2.2. Cell count
-
1.
A 0.4% solution of trypan blue was prepared in PBS.
-
2.
1 volume of trypan blue stock solution was added to 1 volume of cell suspension (dilution factor 1:2).
-
3.
The chambers of the hemocytometer were filled with cell suspension prepared in step 2.
-
4.
The number of bright cells that were not colored blue were counted as viable cells under a microscope at low magnification.
-
5.
Cell number: .
2.2.3. Seeding
Since the cell pellet was re-suspended in 1 ml (1000 μL) of growth media (in step 10 of passaging), to seed 1.3 × 104 cells in each well of the 96 well plates, the further protocol was followed:
-
1.
The amount of cell suspension for each well (A) = .
-
2.
The amount of cell suspension for 96 wells (B) = 96 × A
-
3.
The amount of growth media for each well (C) = 100 μL/well – A
-
4.
The amount of growth media for 96 wells (D) = 96 × C
-
5.
B μL of cell suspension was added to D μL of growth media and the result solution was pipetted.
-
6.
100 μL of the result solution was added to each well of the 96-well plate.
-
7.
The plate was gently shaken and placed in an incubator at 37 °C, 5% CO2, and 95% humidity for 24 h.
2.2.4. Cell cryopreservation (the protocol of cell freezing)
The cultured cells were cryopreserved after 2–4 times of passages according to the following steps:
-
1.
The cells were trypsinized (as it was explained in steps 1 to 8).
-
2.
The cell suspension was transferred to the tube and was gently centrifuged at 1500 rpm for 5 min.
-
3.
After removing the supernatant, the cell pellet was gently and quickly re-suspended in 1 ml freezing media (90% FBS, 10% Dimethyl sulfoxide or DMSO) per vial.
-
4.
The cryotube containing freezing media plus cells was placed in Mr. Frosty ((Nalgene; Nalge Nunc, USA) freezing container.
-
5.
The cryotube was frozen overnight at −80 °C.
-
6.
The vials were transferred to a liquid N2 tank for indefinite storage.
2.2.5. Protocol for thawing frozen cells
-
1.
The cryotube containing the frozen cells was removed from liquid nitrogen storage and immediately placed into a 37 °C water bath.
-
2.
The cells were quickly thawed (<1 min) in the 37 °C water bath until there was just a small bit of ice left in the vial.
-
3.
The thawed cells were transferred dropwise into the centrifuge tube containing 9 ml of pre-warmed complete growth medium in order to dilute the DMSO of the freezing media.
-
4.
The cell suspension was centrifuged at 1500 rpm for 5 min.
-
5.
After removing the supernatant, the cell pellet was re-suspended in a pre-warmed complete growth medium by pipetting.
-
6.
The cells were cultured in appropriate flasks as it was explained in steps 11 to 13 of passaging.
2.3. Extract preparation
The extracts were obtained at a ratio of 3 cm2/ml following ISO 10993-12 recommendations [35]. Therefore, the specimens were fabricated rectangular (2 cm × 1.5 cm × 1 mm) and were cut into two halves to be completely immersed in growth media. They were sterilized by exposure of both faces to ultraviolet irradiation (Noura Layeh Negar Co., Isfahan, Iran) for 40 min (20 min for each side). Since the specimens’ surface area was 6 cm2 (each specimen has two surfaces), each specimen was immersed in 2 ml DMEM with 10% FBS and 1% penicillin-streptomycin and was incubated at 37 °C and 5% CO2 for one day (24 h). After the incubation period, the extracts were removed and filtered through 0.22-μm cells using acetate filters to sterile the tubes. After removing the extracts, the specimens were immersed in new growth media (another 2 ml DMEM with 10% FBS and 1% penicillin-streptomycin) and were incubated at 37 °C and 5% CO2 for two days (48 h). After this incubation period, on the third day of the process, the extracts were removed and filtered to sterile the tubes again. These steps were repeated every 48 h and the extracts were renewed, removed, and filtered on the fifth and seventh days of the process. 5 growth mediums (DMEM with 10% FBS and 1% penicillin-streptomycin) without disks were also incubated similarly to serve as the control groups.
2.4. Evaluation of the cytotoxicity of nanoparticles with the MTT assay
Human Gingival Fibroblasts (HGF) were used to determine the cytotoxic effects of PMMA-containing copper oxide nanoparticles. As explained previously, the number of 1.3 × 104 cells were seeded in each well of 96-well plates (5 wells for the extract of each specimen; therefore 25 wells for each concentration of nanoparticles). The multiwell plates were incubated at 37 °C, 5% CO2 for 24 h as the next step. After seeding the cells for 24 h, the culture medium was removed from the wells and equal volumes (100 μL) of the extracts were added to each well, except for the control wells. In each control well, 100 μL of the incubated growth media (DMEM with 10% FBS and 1% penicillin-streptomycin) was added. Then, 96-well cell culture plates were incubated at 37 °C and 5% CO2 for 24 and 72 h. Following the removal of the test extracts after 24 and 72 h, 75 μL DMEM together with 25 μL of a 2 mg/ml MTT solution (tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide) were added to each well and incubated in a dark environment at 37 °C and 5% CO2 for 3 h to form formazan crystals. After the incubation period, the plates were shaken at 11,100 rpm for 5 min. Then, MTT was aspirated and 100 μL of Dimethyl Sulfoxide (DMSO) was added to each well. Subsequently, the light absorbance was measured at the wavelengths of 570 and 630 nm using a UV–visible spectrophotometer to evaluate the optical density of the wells:
% viability = Optical density of the experimental groups/optical density of the control group × 100.
Days 1, 3, 5, and 7 were the intervals that the culture media were in direct contact with the PMMA discs. PMMA extracts were in direct contact with HGFs on each incubation day for 24 and 72 h and an MTT assay was done after these incubation periods. This means that after day 1, 15 of the PMMA extracts were in direct contact with HGFs, and the cytotoxic analysis was performed after 24 h and 72 h on these extracts. 15 other extracts were placed in contact with HGFs after 3 days, the others after 5 days and a number after 7 days. These extracts were also tested after 24 h and 72 h. The mean MTT cell viability of different CuO concentrations of each group was compared with the controls of the same group. The results of the MTT assay analysis of the 24-h HGF incubation were compared to those of the 72-h incubation period for the first, third, fifth, and seventh days independently.
2.5. Mechanical properties
Flexural strength and surface roughness tests were performed to determine the mechanical properties of the specimens. Also for this, the sample size was calculated using the sample means presented in a similar study with 90% power and α = 0.05 [36]. The sample size was calculated to be at least n = 2 in each group. Therefore, a total of 40 specimens with 65 × 10 × 2.5 mm dimensions were prepared in five groups (n = 8) according to ADA specification No. 12 to gain better results [36]. The process of specimens’ fabrication is explained previously.
The specimens were subjected to a rugosimeter (TESA, rugosurf20, Switzerland) for the evaluation of surface roughness values. Three readings (speed: 0.5 mm/s; distance: 4.8 mm; length reading: 4.0 mm) were made with a cutoff of 0.8 μm in each sample surface using a roughness tester. The first measurement was made on the center mark and the other two were performed at a distance of 5 mm to the left and the right. The average of the three values was calculated.
All specimens were stored in distilled water at 37 °C before the test. The flexural strength test was performed using a universal testing machine (Zwick, Z020, Germany) via three-point loading following ISO 20795-1 [14]. The test was performed at a 5 mm/min crosshead speed until fracture. The distance between the support arms was kept constant at 50 mm. The flexural load was measured at the point of fracture of the specimen in Newton. The ultimate flexural strength was measured using the following formula:
Where (F) indicated the load at fracture in Newton, (I) showed the span length of the specimen between the supporter's arms, (b) was the width of each specimen, and (h) was the thickness of each specimen.
2.6. Microstructure of PMMA-copper oxide nanoparticle samples
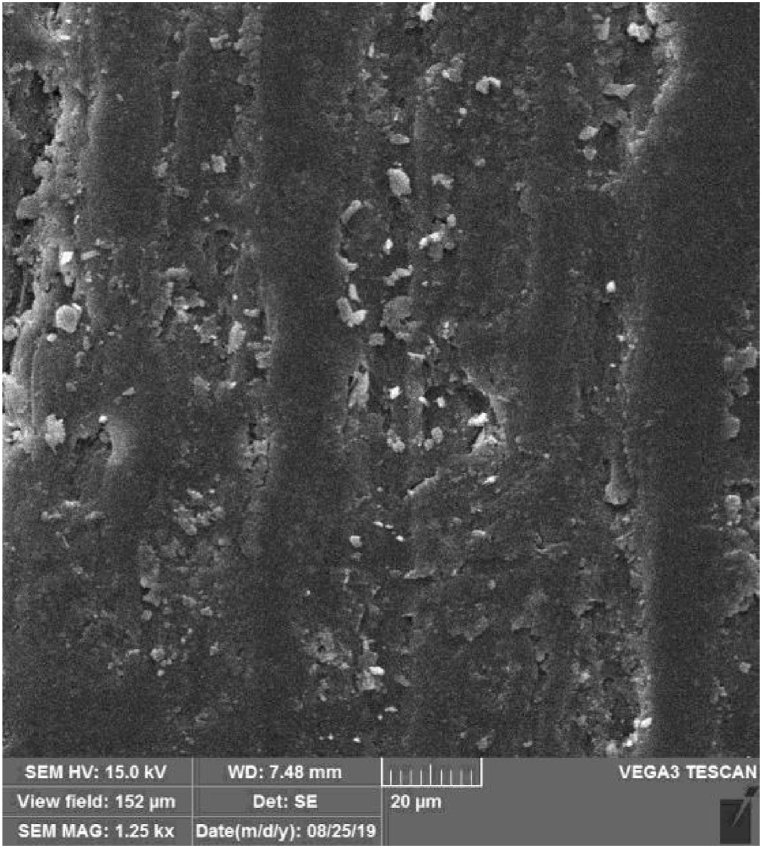
Scanning Electron Microscopy (SEM) was performed on polymerized PMMA-CuO NPs to evaluate the distribution of CuO NPs in the acrylic resin. SEM records information about a sample's surface. Therefore, it can be a valuable tool for comparison of surface distribution in different concentrations of particles. The observations were made using a TESCAN-Vega3 scanning microscope (Tescan Orsay Holding Kohoutovice, Czech Republic). This microscope combines SEM imaging and lives elemental composition analysis in a single window of TESCAN's Essence software. The scanning was carried out after coating the specimens with gold (Quorum, Q150 R ES, UK) at an operating voltage of 15.0 kV. The representative SEM micrographs of the reinforced PMMA specimen were shown at a magnification of ×1.25 k (Fig. 1).
Fig. 1.
Normal distribution of the copper oxide nanoparticles on the surface of the polymethyl methacrylate discs at 50 μg/ml concentration.
2.7. Statistical analysis
The distribution of the data was assessed by the Shapiro-Wilk test. The distribution of the MTT assay data was not normal based on the results of the Shapiro-Wilk test (P < 0.05). Hence, the non-parametric Kruskal-Wallis test and post-hoc Dann's test were used for data analysis. The distribution of the surface roughness and flexural strength was normal (P ≥ 0.05) therefore, one-way ANOVA and post-hoc Tukey's test were used for data analysis. All data analyses were done using the SPSS software version 25 (IBM, Chicago, IL, USA). The MedCalc statistical software version 20.013 (Mariakerke, Belgium) with 90% power and α = 0.05 was used for sample size calculation.
3. Results
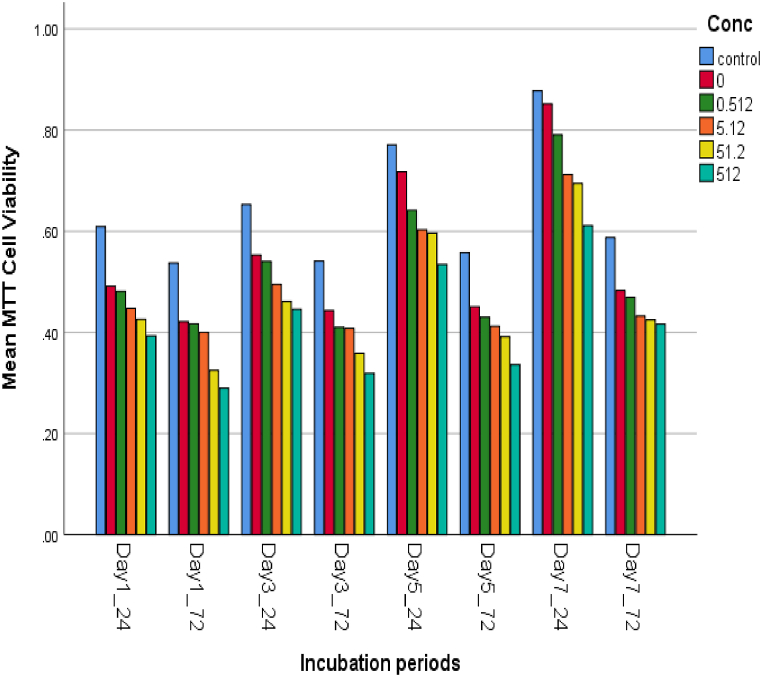
Evaluation of the microstructure of the specimens showed the normal distribution of the NPs in the specimens for all concentrations (Fig. 1). The cell viability percentage at various concentrations of CuO NPs was assessed relative to the incubation period of the NP-containing PMMA discs 24 and 72 h after one, three, five, and seven days of incubation. According to the results of the MTT assay analysis, the survival rates of the cells changed with a dose- and time-dependent pattern (Fig. 2). The cell viability percentage dropped below 70% limitation 24 and 72 h after the first, third, and fifth incubation days at 500 μg/ml NP concentration (65%, 67%, and 68% after 24 h; 52%, 57%, and 60% after 72 h) as well as 72 h after the first and third days of incubation at 50 μg/ml NP concentration (60% and 64%, respectively) (Table 1)The samples containing 500 μg/ml NPs resulted in the lowest percentage of cell viability in all incubation periods, while the highest cell viability was observed 24 h after the seventh day of incubation in NP-free PMMA discs. The viability of the cells in contact with 500 μg/ml of NPs reached 52%–70% after seven days. The comparison of different NP concentrations showed that the NP-free PMMA and the control group (Culture medium with no extract) were not statistically significant (p > 0.05) (Table 1, Table 2). Nonetheless, a significant difference was observed between NPs at 50 and 500 μg/ml concentrations with NP-free PMMA and controls concerning cell viability (p < 0.001). Although all the samples demonstrated an increasing trend of cell viability on the third, fifth, and seventh days, the percentage of cell viability was significantly lower after 72 h than after 24 h in all the incubation periods (p < 0.001). (Table 2).
Fig. 2.
Increasing trend of cell viability and decreasing trend of cytotoxicity over time in different concentrations of copper oxide nanoparticles incorporated in denture base resin. At the same concentrations in the same incubation periods, the percentage of cell viability was significantly lower after 72 h than after 24 h.
Table 1.
Comparison of the cell survival rates (% of optical density of the extract treated cells) to the control group of the treated materials in different incubation periods.
| Incubation period | Nanoparticles concentration (μg/ml) | Mean MTT cell viability | Std. deviation | Cell survival rate (%) |
|---|---|---|---|---|
| Day 1–24✝ | 0 | 0.49 | 0.03 | 81 |
| 0.5 | 0.48 | 0.04 | 80 | |
| 5 | 0.44 | 0.01 | 73 | |
| 50 | 0.42 | 0.01 | 70 | |
| 500 | 0.39 | 0.04 | 65* | |
| Day1–72✝ | 0 | 0.42 | 0.01 | 79 |
| 0.5 | 0.41 | 0.01 | 75 | |
| 5 | 0.39 | 0.01 | 73 | |
| 50 | 0.32 | 0.01 | 60* | |
| 500 | 0.28 | 0.01 | 52* | |
| Day3–24 | 0 | 0.55 | 0.05 | 84 |
| 0.5 | 0.53 | 0.05 | 81 | |
| 5 | 0.49 | 0.03 | 75 | |
| 50 | 0.42 | 0.01 | 70 | |
| 500 | 0.44 | 0.02 | 67* | |
| Day3–72 | 0 | 0.44 | 0.03 | 81 |
| 0.5 | 0.41 | 0.02 | 77 | |
| 5 | 0.40 | 0.01 | 74 | |
| 50 | 0.35 | 0.02 | 64* | |
| 500 | 0.31 | 0.01 | 57* | |
| Day5–24 | 0 | 0.71 | 0.06 | 92 |
| 0.5 | 0.64 | 0.07 | 83 | |
| 5 | 0.60 | 0.03 | 77 | |
| 50 | 0.59 | 0.04 | 76 | |
| 500 | 0.53 | 0.01 | 68* | |
| Day5–72 | 0 | 0.45 | 0.02 | 81 |
| 0.5 | 0.43 | 0.01 | 78 | |
| 5 | 0.41 | 0.02 | 74 | |
| 50 | 0.39 | 0.02 | 70 | |
| 500 | 0.33 | 0.01 | 60* | |
| Day7–24 | 0 | 0.85 | 0.03 | 97 |
| 0.5 | 0.79 | 0.04 | 90 | |
| 5 | 0.71 | 0.03 | 81 | |
| 50 | 0.69 | 0.02 | 79 | |
| 500 | 0.61 | 0.04 | 70 | |
| Day7–72 | 0 | 0.48 | 0.03 | 82 |
| 0.5 | 0.46 | 0.04 | 79 | |
| 5 | 0.43 | 0.01 | 74 | |
| 50 | 0.42 | 0.01 | 72 | |
| 500 | 0.41 | 0.03 | 70 |
*Concentrations at which copper oxide nanoparticles are considered cytotoxic.
✝The cell viability was tested after 24 h and 72 h of contact with HGF cells after each incubation period (days 1, 3, 5, and 7).
Table 2.
P-values of the post –hoc Dunn's test pairwise comparison of various concentrations of copper oxide nanoparticles in polymethyl methacrylate at each incubation period.
| Day/Concentrations (μg/ml) | Day1–24 | Day1–72 | Day3–24 | Day3–72 | Day5–24 | Day5–72 | Day7–24 | Day7–72 |
|---|---|---|---|---|---|---|---|---|
| Control – 0 | 0.187 | 0.176 | 0.446 | 0.702 | 1.000 | 0.217 | 1.000 | 0.95 |
| Control – 0.5 | 0.025a | 0.072 | 0.193 | 0.006a | 0.014a | 0.013a | 0.376 | 0.016a |
| Control – 5 | 0.000a | 0.001a | 0.000a | 0.003a | 0.000a | 0.000a | 0.000a | 0.000a |
| Control – 50 | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a |
| Control - 500 | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a |
| 0–0.5 | 1.000 | 1.000 | 1.000 | 1.000 | 0.679 | 1.000 | 1.000 | 1.000 |
| 0–5 | 0.713 | 1.000 | 0.383 | 1.000 | 0.027a | 0.702 | 0.001a | 0.158 |
| 0–50 | 0.003a | 0.002a | 0.004a | 0.001a | 0.011a | 0.031a | 0.000a | 0.03a |
| 0–500 | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.000a | 0.004a |
| 0.5–5 | 1.000 | 1.000 | 0.826 | 1.000 | 1.000 | 1.000 | 0.273 | 0.667 |
| 0.5–50 | 0.03a | 0.005a | 0.012a | 0.175 | 1.000 | 0.423 | 0.050a | 0.168 |
| 0.5–500 | 0.001a | 0.000a | 0.000a | 0.001a | 0.003a | 0.000a | 0.000a | 0.027a |
| 5–50 | 1.000 | 0.313 | 1.000 | 0.260 | 1.000 | 1.000 | 1.000 | 1.000 |
| 5–500 | 0.92 | 0.004a | 0.353 | 0.001a | 0.150 | 0.010a | 0.150 | 1.000 |
| 50–500 | 1.000 | 1.000 | 1.000 | 1.000 | 0.308 | 0.308 | 0.679 | 1.000 |
Statistically significant difference.
Descriptive statistics of the flexural strength of the tested materials have been illustrated in Table 3. According to the results of one-way ANOVA and post-hoc Tukey's test, a comparison of NP-containing PMMA discs and the control group showed a statistically significant increase in the flexural strength when the discs were fabricated with 50 and 500 μg/ml CuO NPs (p = 0.011 and p = 0.024, respectively). Higher concentrations of NPs resulted in the higher flexural strength of PMMA discs. Nevertheless, the addition of 500 μg/ml of NPs caused a slight decrease in the flexural strength of the discs, but it was not significantly different from the values obtained at the 50 μg/ml NP concentration (p = 0.996).
Table 3.
The effects of various concentrations of copper oxide nanoparticles on polymethyl methacrylate flexural strength according to the results of one-way ANOVA and post-hoc Tukey's tests.
|
Concentration (μg/ml) |
Mean (MPa) | Std. deviation | Std. error | 95% confidence interval for the mean |
Minimum | Post-hoc Tukey's P-value✝ | One-way ANOVA P value | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| 0 | 65.2600 | 13.83358 | 6.18657 | 46.0833 | 80.4367 | 44.10 | 0.005* | |
| 0.5 | 73.2600 | 4.88907 | 2.18646 | 67.1894 | 79.3306 | 65.10 | 0.284 | |
| 5 | 75.2600 | 4.90795 | 2.19490 | 69.1660 | 81.3540 | 68.10 | 0.143 | |
| 50 | 81.3800 | 6.77842 | 3.03140 | 72.9635 | 89.7965 | 72.60 | 0.011* | |
| 500 | 79.6000 | 3.90064 | 1.74442 | 74.7567 | 84.4433 | 73.50 | 0.024* | |
* Statistically significant difference.
✝ Each concentration is compared to the 0 concentration group.
Descriptive statistics of the surface roughness of the tested materials have been illustrated in Table 4. According to the results of one-way ANOVA, although adding 0.5, 50, and 500 μg/ml of CuO NPs reduced the surface roughness of the PMMA discs, this reduction was not statistically significant (p = 0.396).
Table 4.
The effects of various concentrations of copper oxide nanoparticles on the surface roughness of the polymethyl methacrylate samples according to the results of one-way ANOVA.
|
Concentration (μg/ml) |
Mean (mm) | Std. deviation | Std. error | 95% confidence interval for the mean |
Minimum | P value | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| 0 | 1.1996 | .28221 | .12621 | .8492 | 1.5500 | .87 | 0.396 |
| 0.5 | .9644 | .27592 | .12339 | .6218 | 1.3070 | .68 | |
| 5 | 1.3718 | .46372 | .20738 | .7960 | 1.9476 | .70 | |
| 50 | .9608 | .19804 | .08857 | .7149 | 1.2067 | .70 | |
| 500 | .9790 | .27031 | .12089 | .6434 | 1.3146 | .73 | |
4. Discussion
As mentioned previously the addition of metal NPs to PMMA has many benefits [11,12]. In this study, CuO NPs incorporated in PMMA were chosen because they are mainly utilized in hospitals due to their antimicrobial ability to kill more than 99.9% of Gram-positive and negative bacteria within 2 h of exposure if a suitable dose is applied [16]. Therefore, finding an effective dose with the minimum toxicity was the main aim of this study. The concentrations used in this study were chosen according to other studies [22,23]. One study showed that the Minimum Inhibitory Concentration (MIC) for the antifungal effect of CuO NPs was 50 μg/ml. The antimicrobial effect was dose-dependent, with >75% reduction at higher doses (500 μg/ml) [22]. Another study showed considerable antimicrobial effects against S. salivarius, S. sanguis, and C. dubliniensis in PMMA contacting CuO NPs in two different concentrations [23].
To evaluate the cytotoxic effects of CuO-containing PMMA in HGF cells, as an in vitro model, a viability assay was used. To evaluate the effect of CuO-NPs on cell viability, mitochondrial function was employed as a cell viability marker in HGF cell lines. This methodology was used in other studies indicating that mitochondria-mediated apoptosis results when mitochondria undergo permeabilization of the outer mitochondrial membrane, and loss of the electrochemical gradient [37].
Based on the results, the first null hypothesis of the study was partially accepted, because NPs were cytotoxic only at high concentrations. However, the second hypothesis was rejected, since the incorporation of NPs increased the flexural strength. Finally, the third hypothesis was accepted due to the unchanged surface roughness of the PMMA discs as a result of NP incorporation.
According to the results of the MTT assay, the percentages of cell viability were reduced with the increase of concentrations of NPs incorporated in PMMA, respectively 24 h after the first day of incubation. The results also revealed a significant difference between the NPs at 50 and 500 μg/ml concentrations with 0 concentration and the control group regarding the cytotoxicity. This finding revealed the dose-dependent cytotoxic effect of NPs, which has been confirmed by many researchers [28,[32], [33], [34]]. These studies reported a cytotoxic effect for CuO NPs (average size of 50 nm) at >20–25 μg/ml concentrations. In the present study, however, the cytotoxic effect of NPs was observed at concentrations higher than 50 μg/ml. The inconsistency between the results might be due to the use of various cell types and direct exposure of the cells to NPs in the above-mentioned studies. The results of the MTT assay also revealed that PMMA itself showed the potential to reduce the cell viability (down to 79%) probably due to the residual monomer that remained unpolymerized [38]. Based on ISO 10993-5, >30% reduction in cell viability is considered cytotoxic [25]. Since the cell viability percentage of PMMA was higher than 70% in all the incubation periods and the pairwise comparison of NP-free PMMA to the control group was not statistically significant, the reduction percentage by PMMA alone was not considered cytotoxic. This finding was confirmed by the research carried out by Ata et al. [40], which reported heat-polymerized acryl as the least cytotoxic type of acryl in comparison with acetal resin and auto-polymerized acryl. They also obtained comparable MTT assay results, as heat-polymerized acryl reduced the cell viability down to 82% on the first day of incubation, but this percentage increased up to 92% on the seventh day. A part of the reduction in cell viability caused by NP-incorporated PMMA discs might be due to the cytotoxicity of PMMA resins and not the added NPs [39,38]. The results obtained by the NP-free PMMA confirm this statement (Table 1) [40].
Apart from the dose-dependent cytotoxic effect of the NPs, the results of the current study presented a time-dependent cytotoxic pattern, which could be discussed in two separate ways. The cytotoxicity of NPs reduced over time and NPs maintained the percentage of cell viability over 70% at all concentrations after the seventh incubation day. This may be related to leachable NPs constantly segregated through days 1–7. Further studies are indicated to support this explanation. Thus, given the pattern of elution of potential cytotoxic agents, soaking dentures before insertion into the oral cavity may be beneficial [29]. Considering the increased cell viability at 50 μg/ml NPs concentration after three days of incubation, CuO-incorporated acrylic dentures were suggested to be soaked in water for at least three days before use. The effect of elution on reducing cytotoxicity has been previously explained in studies using similar methods of cytotoxic evaluation [[34], [41], [42], [40]]. On the other hand, the cytotoxic effect of NPs significantly increased in all incubation periods when the culture medium was in contact with NP-incorporated PMMA discs for a longer period (72 h versus 24 h). Induction of cytotoxicity in this manner was in accordance with the findings obtained by Cohen et al. [33] and Alarifi et al. [32] on the toxicity of CuO NPs in human skin organ culture and human skin keratinocytes, respectively. This might be associated with the reduction in the pH of the culture medium as a consequence of cellular activity. This assumption was confirmed by the findings of a previous study, which demonstrated an increase in the intracellular dissolution of CuO NPs at lower pH levels of the culture medium [30].
Further investigation of the cytotoxic mechanism of CuO NPs indicated that the release of NPs and transformation into lysosome (a process called the “Trojan Horse” mechanism) could lead to cytotoxic effects in a dose-dependent manner [30,41]. In the present study, the higher cell exposure to NPs after 72 h might have led to a higher lysosomal uptake and cell breakdown followed by a significantly lower percentage of cell viability. To the best of our knowledge, no study has been conducted on the cytotoxic effects of CuO NPs incorporated into denture base materials. Nevertheless, the cytotoxic evaluation of silver NPs fabricated in PMMA after 24 and 72 h showed no significant changes in the cytotoxicity of PMMA + Ag NPs (10–20 nm) [25]. The incorporation of Zn, Ti, and Ag [43] as well as Fe NPs [8] also revealed no adverse effects on the growth of human fibroblasts. Yet, the combination of PMMA and 3% wt of either of these NPs could result in a cell viability of over 80% [42], which was higher than the results obtained in the current study. Considering the abundant resources of Cu and its low cost in comparison to other particles such as Ag, CuO NPs could still be a material of choice in prosthetic dentistry due to their antibacterial, antifungal, and mechanical properties. In addition, CuO NPs are well known as a superconducting agent for heat transfer [30], from which dentists can take advantage of improving the thermal conductivity of acrylic dentures. It has been suggested in the literature that the cytotoxicity of NPs depends on many factors such as the nature of the cells [26], size, shape, and dose of NPs [[31], [32], [33], [34],43], and the type of NPs [42]. Hence, it may not be accurate to generalize the results of studies to different situations.
Evaluation of the mechanical properties of the PMMA samples fabricated with CuO NPs showed that CuO NPs could significantly increase flexural strength when added to the samples. The flexural strength of acrylic denture bases is an extremely important issue that has attracted much attention. ISO 20795–1:2013 - Dentistry International Standards have established the flexural strength for self-curing materials as 60 MPa and for heat curing as 65 MPa. The heat-polymerized PMMA samples without NPs produced a 65 Mpa flexural strength, which was strengthened to 81 and 79 Mpa when 50 and 500 μg/ml NPs were added to the samples, respectively in this study [14]. Studies evaluating how the mechanical properties of the acrylic resin are affected by the addition of different NPs have reported conflicting results [3,36,[44], [45], [46]]. Moreover, none of the studies in the literature has assessed the effect of Cu NPs on the mechanical properties of PMMA. A prior study emphasized that the effect of the incorporation of NPs such as TiO2 and SiO2 on the mechanical properties of acrylic resins was directly correlated to the NPs concentration, with nanocomposite strength decreasing with an increase in the NPs concentration [][45]. However, a recent study indicated that adding SiO2 NPs had no significant effects on the flexural strength of acrylic resin [44]. In the present study, the PMMA flexural strength was higher with the addition of a higher concentration of Cu NPs (50 μg/ml) compared to the lower concentration (0 μg/ml), while the addition of 500 μg/ml Cu NPs slightly reduced the flexural strength. Accordingly, it may be concluded that adding suitable concentrations of Cu NPs to PMMA might enhance the mechanical properties of denture bases in clinical practice. But, it should be noted that one of the problematic issues in incorporating NPs into PMMA is related to the lack of chemical bonds between inorganic materials and PMMA which can be improved by silanization [46].
In the present study, NPs did not significantly change the surface roughness of the PMMA discs. Since the surface roughness test is concerned with the outer surface and not the inner surface of the nanocomposite, when a small percentage of NPs was added to the acrylic resin, only a few particles were involved on the surface of the specimens. These results were in agreement with those of the research carried out by Al-Hiloh et al. [47], in which silanized zirconium oxide was added to acrylic resin. However, some other studies have revealed contradictory results. For instance, Cevik et al. reported that adding silica led to an increase in surface roughness [48], while Aljafery et al. showed a decreasing effect [49].
To note, coupling agents are recommended in the literature for modification of metal oxide NPs. Bio-safe and diacid N-trimellitylimido-l-valine were added to CuO NP's surface to increase the dispersion of NPs into the polymeric matrix [50]. Future studies can focus on such coupling agents.
Unfortunately, due to financial limitations, the current study evaluated the cytotoxicity of CuO NPs through a single test on a certain cell line. Furthermore, the evaluation of mechanical properties was limited to a short incubation period. Also, aging procedures such as thermal cycling were not performed. The use of only one resin and also being an in vitro study can be stated as other limitations of this study.
Hence, future studies are recommended to evaluate the cytotoxicity of CuO NPs using other cytotoxicity tests, such as contact cytotoxic tests, various cell lines, various time exposures, different particle sizes, other resins, thermal conductivity, and chemical analysis using methods such as XRD and FTIR. Moreover, silanization methods are advised in future studies. Assessment of the effects of different coatings on the cytotoxicity of NPs may also be beneficial. The color and esthetic properties of PMMA incorporated by CuO NPs should be considered in upcoming research on this topic. In addition, evaluation of the impact of aging on the mechanical properties of nano-enhanced PMMA can be of particular importance, since the washout of NPs over time may adversely affect the properties of the composite.
5. Conclusion
Within the limitations of this study, the following conclusions can be obtained:
-
1.
The CuO NPs were significantly cytotoxic only when applied at high concentrations (500 μg/ml), but the cytotoxic effect presented a descending trend over time. According to the results, no cytotoxic effect was observed in any of the experimental groups after seven days of incubation.
-
2.
CuO NPs increased the flexural strength at 50 and 500 μg/ml concentrations.
-
3.
The surface roughness of the PMMA discs was not affected by the addition of CuO NPs.
-
4.
CuO NPs can be used in denture base material in moderate concentrations (50 μg/ml) to increase flexural strengths.
Author contribution statement
Elham Ansarifard: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Maryam Zahed: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Negar Azarpira: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Saghar Jooyandeh: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This article was based on the thesis written by Dr. Saghar Jouyande. The authors could like to thank the Vice-Chancellor for Research Affairs of Shiraz University of Medical Science for financially supporting this research (grant No. 97-01-76-18552, ethical code: IR.SUMS.REC.1398.420). They would also like to appreciate Dr. Mehrdad Vosoughi at the Dental Research Development Center of the School of Dentistry for the statistical analysis of the data. Thanks also go to Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for her invaluable assistance in editing the manuscript.
References
- 1.Zarb G.A., Fenton A.H., editors. Prosthodontic Treatment for Edentulous Patients: Complete Dentures and Implant-Supported Prostheses. thirteenth ed./b. Elsevier/Mosby; St. Louis, Missouri: 2013. p. 452. [Google Scholar]
- 2.Balos S., Puskar T., Potran M., Milekic B., Koprivica D., Terzija J., et al. Modulus, strength and cytotoxicity of PMMA-silica nanocomposites. Coatings. 2020 Jun 23;10:583. [Google Scholar]
- 3.Sodagar A., Kassaee M.Z., Akhavan A., Javadi N., Arab S., Kharazifard M.J. Effect of silver nano particles on flexural strength of acrylic resins. Journal of prosthodontic research. 2012;56(2):120–124. doi: 10.1016/j.jpor.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad N., Jafri Z., Khan Z.H. Evaluation of nanomaterials to prevent oral Candidiasis in PMMA based denture wearing patients. A systematic analysis. J Oral Biol Craniofac Res. 2020 Jun;10(2):189–193. doi: 10.1016/j.jobcr.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleiznys A., Zdanavičienė E., Žilinskas J. Candida albicans importance to denture wearers. A literature review. Stomatologija. 2015 Jan 1;17:54–66. [PubMed] [Google Scholar]
- 6.Talattof Z., Azad A., Zahed M., Shahradnia N. Antifungal activity of xylitol against Candida albicans: an in vitro study. J. Contemp. Dent. Pract. 2018 Feb 1;19(2):125–129. doi: 10.5005/jp-journals-10024-2225. [DOI] [PubMed] [Google Scholar]
- 7.Acosta-Torres L.S., Mendieta I., Nuñez-Anita R.E., Cajero-Juárez M., Castaño V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012;7:4777–4786. doi: 10.2147/IJN.S32391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acosta-Torres L.S., López-Marín L.M., Nunez-Anita R.E., Hernández-Padrón G., Castaño V.M. Biocompatible metal-oxide nanoparticles: nanotechnology improvement of conventional prosthetic acrylic resins. J. Nanomater. 2011 Jan 1:2011. [Google Scholar]
- 9.Dutra-Correa M., Leite A.A.B.V., de Cara S.P.H.M., Diniz I.M.A., Marques M.M., Suffredini I.B., et al. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018 Oct 1;77:66–71. doi: 10.1016/j.jdent.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Nam K.Y. Characterization and bacterial anti-adherent effect on modified PMMA denture acrylic resin containing platinum nanoparticles. J Adv Prosthodont. 2014 Jun;6(3):207–214. doi: 10.4047/jap.2014.6.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H.H., Lee J.H., Yang T.H., Kim Y.J., Kim S.C., Kim G.R., Kim H.R., Lee C.J., Okubo C. Evaluation of the flexural mechanical properties of various thermoplastic denture base polymers. Dent. Mater. J. 2018 Nov 27;37(6):950–956. doi: 10.4012/dmj.2017-373. [DOI] [PubMed] [Google Scholar]
- 12.Agnihotri R., Gaur S., Albin S. Nanometals in dentistry: applications and toxicological implications-a systematic review. Biol. Trace Elem. Res. 2020 Sep;197(1):70–88. doi: 10.1007/s12011-019-01986-y. [DOI] [PubMed] [Google Scholar]
- 13.Priyadarsini S., Mukherjee S., Mishra M. Nanoparticles used in dentistry: a review. J Oral Biol Craniofac Res. 2018;8(1):58–67. doi: 10.1016/j.jobcr.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardita A., Ismiyati T., Wahyuningtyas E. Effect of addition titanium dioxide nanoparticles as acrylic resin denture base filler on cytotoxicity. Majalah Kedokteran Gigi Indonesia. 2019;5(2):86–91. [Google Scholar]
- 15.Gad M.M., Al-Thobity A.M. The impact of nanoparticles-modified repair resin on denture repairs: a systematic review. Japanese Dental Science Review. 2021 Nov 1;57:46–53. doi: 10.1016/j.jdsr.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gawande M.B., Goswami A., Felpin F.X., Asefa T., Huang X., Silva R., Zou X., Zboril R., Varma R.S. Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem. Rev. 2016 Mar 23;116(6):3722–3811. doi: 10.1021/acs.chemrev.5b00482. [DOI] [PubMed] [Google Scholar]
- 17.Ishida N., Hosokawa Y., Imaeda T., Hatanaka T. Reduction of the cytotoxicity of copper (II) oxide nanoparticles by coating with a surface-binding peptide. Appl. Biochem. Biotechnol. 2020 Feb 1;190(2):645–659. doi: 10.1007/s12010-019-03108-9. [DOI] [PubMed] [Google Scholar]
- 18.Amiri M., Etemadifar Z., Daneshkazemi A., Nateghi M. Antimicrobial effect of copper oxide nanoparticles on some oral bacteria and Candida species. J Dent Biomater. 2017 Mar;4(1):347–352. [PMC free article] [PubMed] [Google Scholar]
- 19.Grigore M.E., Biscu E.R., Holban A.M., Gestal M.C., Grumezescu A.M. Methods of synthesis, properties and biomedical applications of CuO nanoparticles. Pharmaceuticals. 2016 Nov 30;9(4) doi: 10.3390/ph9040075. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5198050/ [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan S., Ahamed M., Al-Khedhairy A., Musarrat J. Biocidal effect of copper and zinc oxide nanoparticles on human oral microbiome and biofilm formation. Mater. Lett. 2013 Apr 1;97:67–70. [Google Scholar]
- 21.Ramazanzadeh B., Jahanbin A., Yaghoubi M., Shahtahmassbi N., Ghazvini K., Shakeri M., et al. Comparison of antibacterial effects of ZnO and CuO nanoparticles coated brackets against Streptococcus mutans. J. Dent. 2015 Sep;16(3):200–205. [PMC free article] [PubMed] [Google Scholar]
- 22.Ansarifard E., Zareshahrabadi Z., Sarafraz N., Zomorodian K. Evaluation of antimicrobial and antibiofilm activities of copper oxide nanoparticles within soft denture liners against oral pathogens. Bioinorgan. Chem. Appl. 2021;2021 doi: 10.1155/2021/9939275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giti R., Zomorodian K., Firouzmandi M., Zareshahrabadi Z., Rahmannasab S. Antimicrobial activity of thermocycled polymethyl methacrylate resin reinforced with titanium dioxide and copper oxide nanoparticles. International Journal of Dentistry. 2021 Jan 30:2021. doi: 10.1155/2021/6690806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glazkova E., Bakina O., Rodkevich N., Mosunov A., Evstigneev M., Evstigneev V., Klimenko V., Lerner M. Antibacterial properties of PMMA functionalized with CuFe2O4/Cu2O/CuO nanoparticles. Coatings. 2022 Jul 6;12(7):957. [Google Scholar]
- 25.Acosta-Torres L.S., Mendieta I., Nuñez-Anita R.E., Cajero-Juárez M., Castaño V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012;7:4777. doi: 10.2147/IJN.S32391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad J., Alhadlaq H.A., Alshamsan A., Siddiqui M.A., Saquib Q., Khan S.T., et al. Differential cytotoxicity of copper ferrite nanoparticles in different human cells: differential cytotoxicity of copper ferrite nanopaticles. J. Appl. Toxicol. 2016 Oct;36(10):1284–1293. doi: 10.1002/jat.3299. [DOI] [PubMed] [Google Scholar]
- 27.Studer A.M., Limbach L.K., Van Duc L., Krumeich F., Athanassiou E.K., Gerber L.C., Moch H., Stark W.J. Nanoparticle cytotoxicity depends on intracellular solubility: comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010 Sep 1;197(3):169–174. doi: 10.1016/j.toxlet.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Lewinski N., Colvin V., Drezek R. Cytotoxicity of nanoparticles. Small. 2008 Jan;4(1):26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 29.Tornavoi D., Teixeira A., Alves O., Reis A. Cytotoxicity and elemental release of dental acrylic resin modified with silver and vanadium based antimicrobial nanomaterial. J. Health Sci. 2021 Mar 18;23:12–17. [Google Scholar]
- 30.Cronholm P., Karlsson H.L., Hedberg J., Lowe T.A., Winnberg L., Elihn K., Wallinder I.O., Möller L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: a comparison between nanoparticles and their corresponding metal ions. Small. 2013 Apr 8;9(7):970–982. doi: 10.1002/smll.201201069. [DOI] [PubMed] [Google Scholar]
- 31.Ahamed M., Siddiqui M.A., Akhtar M.J., Ahmad I., Pant A.B., Alhadlaq H.A. Genotoxic potential of copper oxide nanoparticles in human lung epithelial cells. Biochem. Biophys. Res. Commun. 2010 May 28;396(2):578–583. doi: 10.1016/j.bbrc.2010.04.156. [DOI] [PubMed] [Google Scholar]
- 32.Alarifi S., Ali D., Verma A., Alakhtani S., Ali B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013 Jul;32(4):296–307. doi: 10.1177/1091581813487563. [DOI] [PubMed] [Google Scholar]
- 33.Cohen D., Soroka Y., Ma’or Z., Oron M., Portugal-Cohen M., Brégégère F.M., et al. Evaluation of topically applied copper(II) oxide nanoparticle cytotoxicity in human skin organ culture. Toxicol. Vitro. 2013 Feb;27(1):292–298. doi: 10.1016/j.tiv.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Hardita A., Ismiyati T., Wahyuningtyas E. Effect of addition titanium dioxide nanoparticles as acrylic resin denture base filler on cytotoxicity. Majalah Kedokteran Gigi Indonesia. 2020;5(2):86–91. [Google Scholar]
- 35.Wallin R.F. MDDI; Los Angeles, CA, USA: 1998. A Practical Guide to ISO 10993-12: Sample Preparation and Reference Materials.https://www.mddionline.com/news/practical-guide-iso-10993-12-sample-preparation-and-reference-materials Available from: [Google Scholar]
- 36.Choi J.J., Uy C.E., Ramani R.S., Waddell J.N. Evaluation of surface roughness, hardness and elastic modulus of nanoparticle containing light-polymerized denture glaze materials. J. Mech. Behav. Biomed. Mater. 2020 Mar 1;103 doi: 10.1016/j.jmbbm.2019.103601. [DOI] [PubMed] [Google Scholar]
- 37.Han J.W., Gurunathan S., Jeong J.K., Choi Y.J., Kwon D.N., Park J.K., et al. Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res. Lett. 2014;9(1):459. doi: 10.1186/1556-276X-9-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ata S.O., Yavuzyılmaz H. In vitro comparison of the cytotoxicity of acetal resin, heat‐polymerized resin, and auto‐polymerized resin as denture base materials. J. Biomed. Mater. Res. Part B: Applied Biomaterials: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2009 Nov;91(2):905–909. doi: 10.1002/jbm.b.31473. https://onlinelibrary.wiley.com/doi/abs/10.1002/jbm.b.31473 Available from: [DOI] [PubMed] [Google Scholar]
- 39.Jorge J.H., Giampaolo E.T., Machado A.L., Vergani C.E. Cytotoxicity of denture base acrylic resins: a literature review. J. Prosthet. Dent. 2003 Aug;90(2):190–193. doi: 10.1016/s0022-3913(03)00349-4. [DOI] [PubMed] [Google Scholar]
- 40.Campos K.D., Viana G.M., Cabral L.M., Portela M.B., Junior R.H., Cavalcante L.M., Lourenço E.J., de Moraes Telles D. Self-cured resin modified by quaternary ammonium methacrylates and chlorhexidine: Cytotoxicity, antimicrobial, physical, and mechanical properties. Dental Materials. 2020;36(1):68–75. doi: 10.1016/j.dental.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Service R.F. Nanotechnology. Nanoparticle Trojan horses gallop from the lab into the clinic. Science. 2010 Oct 15;330(6002):314–315. doi: 10.1126/science.330.6002.314. [DOI] [PubMed] [Google Scholar]
- 42.Chen R., Han Z., Huang Z., Karki J., Wang C., Zhu B., et al. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017 Nov 29;36(6):693–699. doi: 10.4012/dmj.2016-301. [DOI] [PubMed] [Google Scholar]
- 43.Gomes T., Pereira C.G., Cardoso C., Pinheiro J.P., Cancio I., Bebianno M.J. Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquat. Toxicol. 2012 Aug 15;118–119:72–79. doi: 10.1016/j.aquatox.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Al-Thobity A.M., Gad M.M. Effect of silicon dioxide nanoparticles on the flexural strength of heat-polymerized acrylic denture base material: a systematic review and meta-analysis. The Saudi Dental Journal. 2021 Dec 1;33(8):775–783. doi: 10.1016/j.sdentj.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sodagar A., Bahador A., Khalil S., Shahroudi A.S., Kassaee M.Z. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J Prosthodont Res. 2012;57(1):15–19. doi: 10.1016/j.jpor.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Ghaffari T., Hamedi-Rad F. Effect of silver nano-particles on tensile strength of acrylic resins. J Dent Res Dent Clin Dent Prospects. 2015/03/04. 2015;9(1):40–43. doi: 10.15171/joddd.2015.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Hiloh S., Ismail I. A study the effect of addition of silanized zirconium oxide nanoparticles on some properties of high-impact heat-cured acrylic resin. Journal of Baghdad College of Dentistry. 2016 Jan 1;28:19–25. [Google Scholar]
- 48.Cevik P., Yildirim-Bicer A.Z. The effect of silica and prepolymer nanoparticles on the mechanical properties of denture base acrylic resin. J Prosthodont Res. 2016;27(8):763–770. doi: 10.1111/jopr.12573. [DOI] [PubMed] [Google Scholar]
- 49.Aljafery A., Al-Jubouri O., Wally Z., Almusawi R., Abdulrudha N., Haider J. The effects of incorporating Ag-Zn zeolite on the surface roughness and hardness of heat and cold cure acrylic resins. Journal of Composites Science. 2022 Mar 9;6:85. [Google Scholar]
- 50.Mallakpour S., Madani M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coating. 2015 Sep 1;86:194–207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.