Abstract
Background
Pediatric neurorehabilitation has recently employed virtual reality (VR) technologies as a platform to design and implement novel modalities.
Aims
To evaluate the feasibility of a multi-component VR-based program on motor skills and functional postural control for children with hemiplegic cerebral palsy (HCP).
Methods
A single-case-experimental design was conducted on eight children with HCP (12.33 ± 4.71 years and GMFCS= II, I). The VR-based program consisted of 3 sessions per week for four weeks. Timed Up and Go (TUG) test, Functional Reach Test (FRT), Pediatric Balance Scale (PBS), Activities Scale for Kids (ASK), ABILHAND-Kids, and Box and Block Test (BBT) were used to evaluate functional changes.
Outcomes and results
Statistical analysis showed that improvements in functional postural control were significant on at least one balance measure for seven out of eight participants during the intervention phase. For all participants, a significant increase was observed in the BBT scores. Before-after intervention analysis revealed statistically significant improvements in PBS (z = −2.52, p ≤ 0.01), ABILHAND-Kids (z = −2.25, p ≤ 0.01), and ASK (z = −2.38, p ≤ 0.01).
Conclusions and implications
This study provided early evidence of the effectiveness of the multi-component VR-based program in children with HCP. However, future studies with randomized controlled trial design are needed to evaluate the long-term effects and compare them with conventional rehabilitation practice.
Keywords: Cerebral palsy, Hemiplegia, Virtual reality, Postural control, Activities of daily living
1. Introduction
Cerebral palsy (CP) is a common disorder in pediatrics that occurs due to a non-progressive lesion of the immature brain and results in limitations in gross motor function, manual ability, self-care, and daily activities [1,2]. More than thirty percent of children with CP experience motor disorders on one side of the body, known as hemiplegic cerebral palsy (HCP) [3]. Although 99% of children with HCP are capable of independent ambulation without assistive devices such as wheelchairs [3], they have deficits in dynamic postural control and functional balance [4]. In addition to balance deficits, upper extremity dysfunction has been identified as a substantial problem in children with HCP. Inability to grip, poor eye-hand and bimanual coordination, abnormal upper extremity posture, weak in-hand manipulation, and sensory processing problems are commonly evident in these children [5]. Therefore, functional ability of the upper extremities and whole body postural control are necessary for children with HCP to be able to participate in daily activities [6].
Physical and occupational therapists use diverse approaches and strategies to manage muscle tone and facilitate functional motor performance and participation in daily activities such as neurodevelopmental treatment, positioning, constraint-induced movement therapy (CIMT), bimanual therapy, goal-directed training, orthotics, and electrical stimulation [[7], [8], [9]]. However, pediatric neurorehabilitation has recently employed virtual reality (VR) technologies as a platform to design and implement novel modalities to provide a beneficial method for rehabilitation practitioners. VR has impressive features that provide a safe environment to engage children in an enjoyable learning process by generating computer-based interactive games/tasks [10]. There is a wide spectrum of VR technologies that are used in rehabilitation, from full-immersive VR to non-immersive video games. Non-immersive technologies are more available systems that are commonly used for children with CP. Several randomized controlled trial (RCT) studies exhibited that non-immersive VR intervention can lead to positive outcomes in children with HCP. These studies reported that VR-based group had more significant improvements in gross motor function, independence in daily activities [11], upper extremity function, active joint range of motion, quality of life [12], and occupational performance [13] compared to the control group.
Most VR technologies are based on commercial equipment without adequate flexibility to adjust the game parameters according to the children's needs and therapeutic goals [14]. Besides, these systems typically focus on only one aspect of motor function. However, clinical evidence supports multi-component training programs to attain significant improvements in motor skills and postural control [15]. Elnaggar et al. showed that a combination of plyometric and balance exercises improved postural control and muscle strength more than a single-component training program in children with HCP [15]. Similarly, another study embedded five different components in a daily multi-component program including bimanual play, reaching tasks, wearing soft-constraint on the less-affected hand, sensory-motor training, and parent education. The results of this study demonstrated that the multi-component group had more improvements in somatosensory processing compared to the bimanual play group in children with HCP [16]. Therefore, it is hypothesized that developing a customized VR-based program and addressing different motor components, such as dynamic balance, static postural control, and upper extremity movements, would enhance the effectiveness of the VR program.
The International Classification of Functioning, Disability and Health - Children and Youth (ICF-CY) is a biopsychosocial model that focuses on interactions between, body functions and structures, activities and participation, and contextual factors of children and young individuals. Health-related researchers use the ICF-CY model to report their research findings and guide practitioners in the case of selecting outcome measures and planning specific interventions for their clients [17]. So far, no study has used this model to report the effectiveness of VR-based interventions in children with CP [18]. On the other hand, existing studies have focused on the activity subdomain, and the area of participation has been addressed in fewer studies [19]. Therefore, the goal of this study was to investigate the effects of a multi-component VR program on activity and participation domain of ICF-CY in children with HCP. The findings of this study help physical and occupational therapists to find out whether the designed VR system is feasible and effective for improving the activity and participation of children with HCP.
2. Material and methods
2.1. Study design
This study has an AB single-case experimental (SCE) design, which was done across eight participants with one week of baseline and four weeks of intervention phases. This type of experimental study uses repeated and intensive evaluations to map the change of dependent variables for each participant to investigate the effectiveness of the applied interventions in clinical settings [20]. The ethical approval was obtained from Shahid Beheshti University of Medical Sciences Research Committee (No IR.SBMU.RETECH.REC.1401.452).
2.2. Participants
Eight children with HCP were recruited from the CP clinic of Mofid Children's Hospital in Tehran. The following inclusion and exclusion criteria were used for identifying eligible participants: 1) A HCP diagnosis based on pediatric neurologist examination, 2) Gross Motor Function Classification System (GMFCS) Level I and II, 3) Manual Ability Classification System (MACS) Level I and II, 4) A minimum age of 7 years, 5) Modified Ashworth ≤2, 6) Lack of botulinum toxin injection for six months, 7) Mini-mental state examination score ≥24, and 8) Lack of visual and auditory impairments. Participants were excluded if they exhibited poor language skills or insufficient interaction with digital equipment. We outlined the details of the study process for parents and their children and then received the signed informed consent form before commencing the study.
2.3. Intervention
2.3.1. VR system
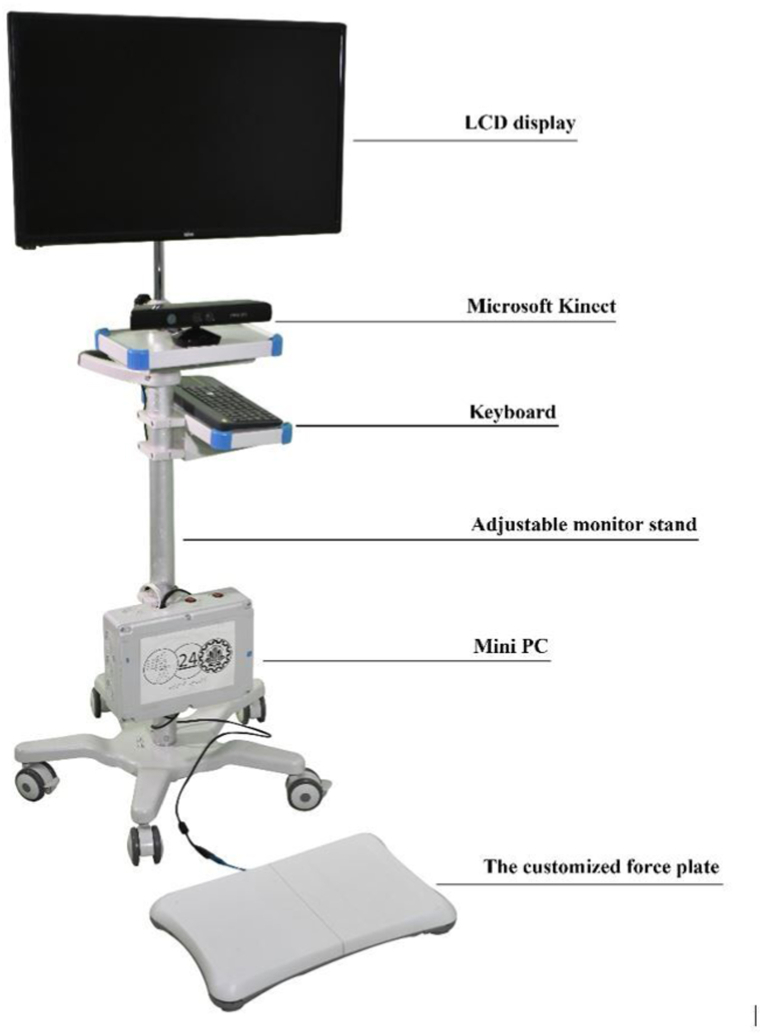
Our non-immersive VR system consists of a VivoMini UN65U Asus, Xbox 360 Kinect sensor, a customized force plate, a 32-inch LCD, a keyboard, and newly designed software with various game scenarios [21] (see Fig. 1). In the treatment sessions, children could see their avatar on the LCD using the motion tracking technology (Kinect Microsoft) and engage in tasks in an attractive virtual environment. Three main types of motor tasks, including reaching, tracking, and holding/dragging targets in multidirectional and diagonal patterns were used for upper extremities training. In addition, dynamic and static balance training including lateral side walking, bending, knee skipping, kicking, random control, limit of stability, and maze control were used to facilitate participants’ postural control. The system provided feedback (explicit, implicit, and continuous), the massed practice, a progress difficulty level, and just-right-challenge patient-specific tasks to focus on the more-affected upper extremity. Some examples of the tasks are presented in Appendix 1.
Fig. 1.
Virtual reality system.
2.3.2. Treatment program
Each session started and finished with 5 min of warm-up and cool-down exercises, respectively including muscle self-stretching, hand weight bearing in sitting condition, trunk rotation, and reaching 75% of maximum capacity. The level of difficulty in treatment sessions was set in accordance with the children's early capacity level and progressed with achieving at least 75% of the total session score. A treatment session contained three parts: (1) upper extremity: 30 min of reaching and tracking toward targets in vertical, horizontal, symmetrical, and asymmetrical directions; (2) static postural control: 15 min of center of pressure exercises including random control, maze control, and limits of stability, (3) dynamic balance: 15 min of dynamic mobility tasks including reaching while lateral side walking, knee skipping, and kicking. The focus of all tasks was on the more-affected side, and the progress of the task difficulty was applied by decreasing the size of targets, reducing the available time to reach the targets, and increasing the distance of the targets. Additionally, task complexity was increased each week for upper extremities and dynamic balance tasks. For example, in the third week, we asked the participants to keep their feet together when doing reaching tasks (see Table 1).
Table 1.
Task complexity.
| Week | Condition | Direction of reaching pattern | Foot placement |
|---|---|---|---|
| First | Sitting on a chair | Vertical/horizontal | – |
| Second | Standing | Vertical/horizontal/diagonal with symmetrical pattern | Comfortable position |
| Third | Standing | Vertical/horizontal/diagonal with symmetrical and asymmetrical patterns | Feet together (Narrow base of support) |
| Fourth | Standing | Vertical/horizontal/diagonal with symmetrical and asymmetrical patterns | Tandem position if possible |
2.3.3. Dosage
Initially, one training session was considered to familiarize children with the protocol of intervention and game scenarios. The dosage of the VR program was 60 min, three times a week for 4 weeks, in a total of 12 treatment sessions. An experienced occupational therapist in the field of pediatric rehabilitation performed all assessments and monitored VR sessions.
2.4. Activity and participation outcome measures
Since the process of clinical measurement was time-consuming and the changes were expected to be established gradually, two data collection sessions per week were done for three outcome measures including Timed Up and Go (TUG) test, Functional Reach Test (FRT), and Box and Block Test (BBT). Other measures including Activities Scale for Kids (ASK), ABILHAND-Kids, and Pediatric Balance Scale (PBS) were administered pre-and post-intervention. We used three different balance tests to detect changes in different aspects of functional postural control such as anticipatory postural adjustment, limit of stability, and sensory strategies [22]. All outcome measures were labeled based on the ICF-CY model.
2.4.1. TUG
The TUG test is a valid and reliable test for measuring control of dynamics during walking as well as anticipatory postural adjustment in children with CP [22]. The process of the TUG test includes rising from a chair, walking 3 meters at a comfortable speed to reach a marker, then turning up to continue walking in the direction of the starting point and sitting down in the chair. The elapsed time to complete the test is recorded as the test score. There was a strong relationship between TUG and PBS scores (r = −0.88, P < 0.01) and high test-retest reliability in children with CP (ICC = 0.99) [23,24]. The TUG test has been shown to have minimum clinically important difference (MCID) values of 1.1–1.7 and 0.7–1.2 for children with CP at GMFCS levels I and II, respectively [25]. This test covers the b770, d4103, and d4104 codes of ICF-CY.
2.4.2. FRT
The FRT was developed to measure the orientation in space and maximum stability limits during standing conditions for children with CP [22]. To perform FRT, the participants were instructed to raise their less-affected arm at the level of the shoulder, and then reach forward as far as they can without the heel rising, concurrently. The maximum distance was recorded. This test was repeated three times and the average was considered as the final test score. Previous studies have shown that FRT has excellent test-retest reliability (ICC = 0.95) and good validity with TUG scores (r = −0.77, P < 0.01) in children with CP [22,23]. This test covers the d4106 and d4452 codes of ICF-CY.
2.4.3. PBS
The PBS is a modified version of the Berg Balance Scale adapted to apprise sensory strategies, as well as other aspects of balance and postural control during functional tasks in children with CP [22]. The PBS consists of 14 items, each item is scored on a 5-point scale, with a maximum score of 56 indicating excellent balance. This test showed strong concurrent validity with the Gross Motor Function Measure-66 (r = 0.94, P < 0.01) and high test-retest reliability (ICC = 0.99) in children with CP [26,27]. The MCID for the PBS has been estimated to be between 3.66 and 5.83, which can be used to detect clinically significant changes in functional balance over time [27]. This test covers d4103-d4106, d4153, d4154, d4200, and d4452 codes of ICF-CY.
2.4.5. BBT
The BBT is a functional test commonly used to appraise manual dexterity in children with CP. This test uses a rectangular wooden box divided into two compartments with equal size and 150 blocks. To perform this test, participants were instructed to transfer blocks from one compartment to the other. The number of transferred blocks in 1 minute was recorded as the test score. The BBT has high test-retest reliability (ICC = 0.98) and strong construct validity with the Melbourne Assessment 2 (r = 0.78, P < 0.01) in children with CP [28]. The MCID for the BBT has been estimated to range from 5.29 to 6.46, so minimum improvements in this range indicate a clinically significant change for children with HCP [28]. This test covers d440 and d445 codes of ICF-CY.
2.4.6. ABILHAND-Kids
This test is a parent's report questionnaire with 21 items for determining hand function in children's daily life based on difficulty (impossible/difficult/easy). Excellent validity and reliability of the Persian version of ABILHAND-Kids in children with CP have been reported [29]. ABILHAND-Kids scores were calculated and reported in logit-based units. This test covers d4-d5 codes of ICF-CY.
2.4.7. ASK
ASK consists of 30 items that measure physical performance in a broad range of activities of daily living including dressing, personal care, locomotion, play, standing skills, transfers, and other skills. This test has been shown to have good validity and reliability in children with CP [30]. This test covers d2, d4, d5, d6, d8, d9 codes of ICF-CY.
2.5. Data analysis
2.5.1. Visual analysis
First, we applied visual analysis [31] as an acceptable approach among neurorehabilitation researchers for presenting stability in the baseline phase, trend line, and then examining the mean difference across two phases. All graphs were illustrated using GraphPad Prism version 8.4.2 for Windows (GraphPad Software, La Jolla California USA). We used 25% of the median for determining stability in the baseline phase (80% of data points should fall within this range). We also assessed the clinical significance of observed changes based on the MCID, by calculating the difference between pre- and post-intervention for each participant for BBT, PBT, and TUG.
2.5.2. Statistical analysis
Tau-U, which is a type of rank correlation coefficient, was used to determine the treatment effects on between-phase differences [32]. If there was a baseline trend, residuals from the Theil–Sen regression were used to remove the significant monotonic trend from the baseline phase [33]. Finally, an improved rank correlation effect size, called corrected baseline Tau analysis, was conducted to indicate the strength and direction of treatment effects [33]. The corrected baseline Tau analysis is a non-parametric statistic introduced by Tarlow to resolve the problem of the significant baseline trend. A free online calculator was used for Tau-U analysis [34]. The absolute value of Tau effect size, up to 0.8, was interpreted as very large, 0.6–0.8 as large, 0.2–0.6 as medium, and less than 0.2 as small effectiveness. A simple regression was implemented to illustrate the fit line and identify the phases’ slope. The Wilcoxon test was used for pairwise comparison of before and after intervention test scores.
3. Results
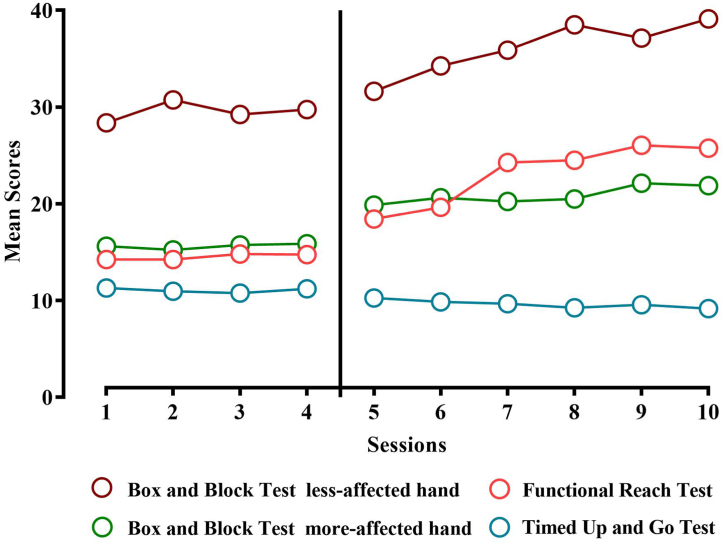
Three girls and five boys, with a mean age of 12.33 ± 4.71 years were recruited. Participants’ characteristics are presented in Table 2. The stability of the baseline time series was confirmed by visual analysis. A baseline trend was found in TUG data for P3, so in this case corrected Tau-U analysis was used. The progress of mean score changes for BBT, FRT, and TUG are illustrated in Fig. 2. Visual representation of progress for each participant is presented in Appendix 2. All participants completed the same dosage of the multi-component VR program (60 min × 3 sessions per week × 4 weeks) with personalized difficulty levels. The number of reaching repetitions gradually increased from an average of 384 ± 35 on the first session to 954 ± 78 on the last session of the program. The mean of overall reaching repetitions was 701 ± 181.
Table 2.
Participants characteristics.
| Participants | Age (Year, Month) | Height (cm)/Weight (kg) | Sex | More-affected side | GMFCS | MACS |
|---|---|---|---|---|---|---|
| P1 | 17 y, 3 mo | 157/50 | Female | Left | II | II |
| P2 | 13 y, 8 mo | 158/52.8 | Female | Right | II | I |
| P3 | 7 y | 117/32.5 | Male | Left | I | I |
| P4 | 7 y, 2 mo | 122/29.5 | Male | Right | II | II |
| P5 | 18 y, 5 mo | 161/41 | Female | Right | I | II |
| P6 | 12 y, 10 mo | 168/83 | Male | Left | I | II |
| P7 | 10 y, 8 mo | 134/25 | Male | Right | I | II |
| P8 | 7 y | 113/20 | Male | Left | II | II |
GMFCS: Gross Motor Function Classification System.
MACS: Manual Ability Classification System.
Fig. 2.
The progress of mean score changes for BBT, FRT, and TUG.
Visual inspection of FRT scores showed a tendency for significantly improved functional balance during the intervention phase. Ascending trends and increasing mean FRT scores, during the intervention phase, were found in all participants. Statistical analysis was in agreement with visual analysis. Except P2 and P7, all participants indicated statistically significant changes in the FRT between two phases that were categorized in large effect size by the Tau-U values. Based on visual analysis, a descending trend of TUG was found under the intervention phase in P1, P4, P5, P7, and P8. The TUG mean score decreased in all participants when compared to the baseline but the change was not statically significant in most cases (P1, P2, P3, P5, P6, and P8). The Tau-U statistics showed a significant between-phase change only in two out of eight participants (P4 and P7) (see Table 3). In addition, PBS mean scores changed significantly from 48 ± 4 pre-intervention to 52.87 ± 3.27 post-intervention (z = −2.52 p ≤ 0.01).
Table 3.
Results of the clinical tests.
| Outcome measures | Mean (SD) |
Tau−U |
Slopes β |
Clinical significance based on MCID | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline phase | Intervention phase | Effect size | SETau | p-value | Baseline phase | Intervention phase | |||
| P1 | BBT more-affected hand | 12.75 (0.95)† | 17.33 (1.03) | 0.765 | 0.288 | ≤ 0.01 | −0.50 | −0.11 | ✓ |
| BBT less-affected hand | 21.75 (0.50)† | 25.5 (2.73) | 0.816 | 0.258 | ≤ 0.01 | 0.30 | 1.28 | ✓ | |
| FRT | 10.75 (0.50)† | 28.33 (4.30) | 0.765 | 0.288 | ≤ 0.01 | 0.10 | 1.91 | NA | |
| TUG | 13.80 (0.58)† | 10.44 (0.69) | −0.443 | 0.382 | 0.12 | −.031 | −0.16 | ✓ | |
| P2 | BBT more-affected hand | 19.25 (0.50)† | 22.67 (1.86) | 0.749 | 0.283 | ≤ 0.01 | −0.10 | −0.74 | ✗ |
| BBT less-affected hand | 28.00 (1.63)† | 33.67 (2.42) | 0.752 | 0.281 | ≤ 0.01 | 0.40 | 1.02 | ✓ | |
| FRT | 14.63 (1.49)† | 18.38 (3.29) | 0.506 | 0.368 | 0.08 | 0.55 | 1.25 | NA | |
| TUG | 9.06 (0.55)† | 8.20 (0.25) | −0.373 | 0.396 | 0.20 | 0.27 | −0.07 | ✓ | |
| P3 | BBT more-affected hand | 18.75 (0.95)† | 23.50 (1.51) | 0.760 | 0.277 | ≤ 0.01 | 0.50 | 0.08 | ✗ |
| BBT less-affected hand | 21.75 (2.50)† | 28.33 (2.42) | 0.709 | 0.301 | ≤ 0.01 | −0.90 | 1.02 | ✓ | |
| FRT | 14.75 (0.95)† | 18.08 (2.40) | 0.690 | 0.309 | ≤ 0.01 | −0.10 | 1.27 | NA | |
| TUG‡ | 10.44 (0.16)† | 10.37 (0.66) | 0.542 | 0.358 | 0.06 | −0.07 | −0.01 | ✗ | |
| P4 | BBT more-affected hand | 21.50 (1.00)† | 27.67 (2.87) | 0.765 | 0.288 | ≤ 0.01 | 0.60 | 1.14 | ✓ |
| BBT less-affected hand | 27.25 (0.25)† | 32.17 (2.13) | 0.762 | 0.290 | ≤ 0.01 | −0.10 | 0.65 | ✗ | |
| FRT | 15.88 (0.62)† | 24.25 (4.31) | 0.747 | 0.297 | ≤ 0.01 | −0.25 | 1.87 | NA | |
| TUG | 10.59 (0.77)† | 8.67 (0.71) | 0,669 | 0.332 | 0.02 | 0.43 | −0.32 | ✓ | |
| P5 | BBT more-affected hand | 19.00 (1.82)† | 28.33 (2.50) | 0.756 | 0.293 | ≤ 0.01 | 0.6 | 1.02 | ✓ |
| BBT less-affected hand | 41.00 (1.15)† | 50.50 (4.03) | 0.756 | 0.293 | ≤ 0.01 | 0.00 | 1.91 | ✓ | |
| FRT | 10.38 (1.79)† | 23.33 (1.96) | 0.756 | 0.293 | ≤ 0.01 | 0.55 | 2.01 | NA | |
| TUG | 10.90 (0.75)† | 9.98 (0.45) | 0.548 | 0.374 | 0.07 | −0.21 | −.0.14 | ✓ | |
| P6 | BBT more-affected hand | 17.50 (2.51)† | 22.50 (2.16) | 0.724 | 0.308 | ≤ 0.01 | −0.20 | 0.65 | ✗ |
| BBT less-affected hand | 47.25 (2.87)† | 52.67 (4.32) | 0.504 | 0.386 | 0.10 | 1.70 | 1.94 | ✓ | |
| FRT | 18.00 (0.81)† | 25.75 (5.40) | 0.716 | 0.312 | ≤ 0.01 | 0.60 | 2.58 | NA | |
| TUG | 10.17 (0.61)† | 9.44 (0.40) | 0.487 | 0.391 | 0.11 | −0.32 | −0.02 | ✗ | |
| P7 | BBT more-affected hand | 11.50 (1.00)† | 18.17 (2.63) | 0.767 | 0.247 | ≤ 0.01 | 0.20 | 1.28 | ✓ |
| BBT less-affected hand | 30.00 (0.00)† | 36.17 (4.95) | 0.574 | 0.349 | 0.05 | 0.00 | 2.08 | ✓ | |
| FRT | 18.75 (0.50)† | 20.67 (1.96) | 0.570 | 0.367 | 0.07 | 0.10 | 0.74 | NA | |
| TUG | 11.93 (0.52)† | 9.18 (0.84) | 0.730 | 0.306 | ≤ 0.01 | −0.23 | −0.40 | ✓ | |
| P8 | BBT more-affected hand | 4.75 (0.50)† | 6.83 (1.16) | 0.719 | 0.296 | ≤ 0.01 | −0.10 | 0.02 | ✗ |
| BBT less-affected hand | 19.25 (1.50)† | 29.67 (2.65) | 0.765 | 0.288 | ≤ 0.01 | 0.70 | 1.20 | ✓ | |
| FRT | 10.00 (0.0)† | 14.67 (2.42) | 0.795 | 0.271 | ≤ 0.01 | 0.00 | 0.80 | NA | |
| TUG | 11.68 (0.47)† | 10.78 (0.91) | 0.487 | 0.39 | 0.11 | 0.17 | −0.40 | ✓ | |
BBT: Box and Block Test, TUG: Timed Up and Go, FRT: Functional Reach Test, MCID: Minimum Clinically Important Difference, ✓: Clinically significant ✗: Not clinically significant, NA: Not Applicable (There is no established MCID for the FRT), †: Stable phase, ‡: Corrected baseline Tau analysis was used, and SE: Standards error. *:P ≤ 0.05 was considered significant.
Visually analysis of data points revealed that the mean level of BBT scores increased through the intervention phase compared to baseline in all participants. Although the baseline phase was stable in all cases, data variability increased in the intervention phase in P3, P4, P5, P6, and P7 with an ascending trend. Results of Tau-U statistical analysis are commensurate with visual analysis and showed a significant improvement in manual dexterity of both more-affected hand and less-affected hand based on the BBT data points. The effect size for the more-affected hand ranged from 0.7 to 0.8, indicating a large effect size. The effect size in the less-affected hand of P1 was 0.81, which is very large, and it was in a medium spectrum for P6 and P7, and large for rest of the participants (see Table 3).
Wilcoxon tests showed that the participants had significantly greater scores post-intervention than baseline assessment in ABILHAND-Kids (z = −2.25, p ≤ 0.01) and ASK (z = −2.38, p ≤ 0.01). Table 4 represents scores for each participant in the baseline and intervention phases.
Table 4.
Results of pairwise comparison for before-after measures.
| Participants | Statistical significance |
Clinical significance based on MCIDa |
|||||
|---|---|---|---|---|---|---|---|
| PBS |
ASK |
ABILHAND-Kids |
PBS | ||||
| Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | Pre-intervention | Post-intervention | ||
| P1 | 47.00 | 51.00 | 59.16 | 78.33 | −1.09 | 0.73 | ✓ |
| P2 | 43.00 | 56.00 | 69.16 | 79.16 | 1.71 | 2.33 | ✓ |
| P3 | 44.00 | 53.00 | 71.66 | 85.83 | 1.31 | 2.79 | ✓ |
| P4 | 48.00 | 53.00 | 56.66 | 71.66 | −1.85 | 1.11 | ✓ |
| P5 | 54.00 | 56.00 | 72.5 | 84.16 | 1.31 | 2.79 | ✗ |
| P6 | 52.00 | 53.00 | 88.33 | 95.83 | 3.21 | 3.53 | ✗ |
| P7 | 51.00 | 55.00 | 95 | 90.83 | −0.19 | 1.31 | ✓ |
| P8 | 45.00 | 46.00 | 42.5 | 49.16 | −0.73 | −0.37 | ✗ |
| Mean (SD) | 48 (4) | 52.87 (3.27) | 69.37 (16.98) | 79.37 (14.34) | 0.46 (1.69) | 1.77 (1.29) | |
| Wilcoxon Z-score (P-value) | −2.52 (≤0.01) | −2.38 (≤0.01) | −2.25 (≤0.01) | ||||
PBS: Pediatric Balance Scale.
ASK: Activities Scale for Kids.
MCID: Minimum Clinically Important Difference.
✓: Clinically significant ✗: Not clinically significant.
There is no established MCID for the ABILHAND-Kids and the ASK.
4. Discussion
This study aimed to explore the effectiveness of a multi-component VR program for children with HCP. Our results showed that hand function, functional balance, and activities of daily living were improved in children with HCP following VR sessions. Although the upper extremity tasks focused on proximal joints, including the shoulder and elbow, results showed that hand skills and manual dexterity in the more-affected hand improved as well. Previous studies that used similar Kinect-based VR interventions have reported improvements in the BBT scores [35] and kinematic hand motion parameters [36] following intervention in children with HCP. These results are in agreement with the findings of a cross-sectional study on individuals with hemiparesis [37]. This study found a correlation between the more-affected hand function and active range of motion of proximal segments and suggested that improving the stability and motor control of the more proximal segments seem to be necessary for the promotion of hand skills [37]. For instance, a clinical study showed hand function improvements after an electrical stimulation combined with vibration by a reaching robot on the patients’ hemiparetic shoulder. It concluded that improvements in proximal regions provide a stabilized base for manipulating objects in daily activities [38]. Moreover, the results of the present study demonstrated that less-affected hand movements improved following the intervention phase. This finding appears to be due to the effects of bilateral motor tasks. Dexterity and fine motor skills in dominant hand of children with HCP are poorer than their peers because of impaired eye-hand coordination [39,40] therefore, we used bimanual tasks to engage less-affected upper extremity and provided multi-sensory feedback during VR sessions to enable children to correct their movement errors over repetitive task practice.
Repetition is a principal part of common rehabilitation approaches for children with HCP, such as CIMT and bimanual training. Results of a clinical study showed that children with HCP repeated tasks with a mean of 365 ± 165 times in CIMT and 285 ± 103 times in bimanual training sessions, and showed that repetition as a key element is correlated with higher motor skill improvements [41]. Shih et al. reported higher repetition in their study. They used a VR-based CIMT program and showed that 853 repetitions of reaching per session in the VR-based CIMT group led to the same motor skill improvement as conventional CIMT. Similar to previous studies, repetitive practice of motor tasks was one of the main features of our VR program, and participants repeated considerably reaching toward targets in different directions during both dynamic and static postural control (a mean of 701 ± 181 times). Shih et al. suggested that using VR technologies in rehabilitation programs can increase participants' motivation, active participation, and the amount of task practice in children with CP [42]. The pertinent literature has revealed that neural repair depends on the existence of task practice. In this regard, Bleyenheuft et al. reported increasing fractional anisotropy and mean diffusivity in corticospinal tract fibers of both affected and unaffected hemispheres after motor skill training in children with HCP and found that these changes correlated with increasing hand function [43]. Therefore, VR technologies may be ideal for planning rehabilitation programs that directly address neural organizations through massed practice [44].
Our findings showed that functional balance improved significantly following the intervention. This is consistent with the findings of previous studies. The results from Pourazar et al. showed improvement in the Y balance test scores in children with HCP aged 7–12 years following 20 sessions of a commercial Kinect dance game [45]. Another study compared traditional occupational therapy intervention with a non-immersive VR intervention in terms of their effectiveness on motor function and activities of daily living. The results of this study revealed that both interventions improved outcome measures, but the non-immersive VR led to more positive changes in all subscales of the Bruininks-Oseretsky Test of Motor Proficiency-Short Form [11]. These results suggest that the used motor tasks such as reaching or tracking in forward, lateral, and diagonal patterns facilitate whole-body coordination and postural stability during functional tasks. Some studies examined the center of pressure in children with CP during reaching tasks and showed that the severity of manual deficits is associated with larger compensatory adjustments and postural instability during reaching [46,47]. This evidence recommends that the content of rehabilitation programs should be directed towards enhancing postural control through upper body tasks such as reaching [48].
In addition, these changes may be justified by the beneficial effects of the postural tasks using the force plate. Tarakci et al. investigated the effectiveness of the Wii balance board on functional balance in children with HCP and reported that FRT and TUG scores improved significantly [49]. These tasks induce active weight shifting and weight bearing on the more involved lower limb in various directions and provide an opportunity for neuromuscular coordination. The theory of motor control suggests that anticipatory postural control should be considered as one of the main concepts of balance training during rehabilitation practice [50]. During these tasks, children were encouraged to shift their center of pressure based on visual feedback, then anticipate the requisites to adopt postural adjustments and maintain their postural stability. As a result, static balance training modulates anticipatory postural muscle activity in children with HCP.
During our VR program, participants performed two tasks simultaneously, for instance, reaching was combined with postural control. These states are defined as dual-task conditions, and the observed improvements can be justified on the basis of dual-task paradigm [51]. Dual-task training provides the opportunity to practice real-life events and enhance the capacity of cognitive processing during secondary motor tasks. Indeed, children should dedicate their cognitive capacity to organize and process two tasks concurrently, which may activate other alternate neural pathways [52]. Therefore, improvement in dual-task functions can lead to better performance in other activities of daily living.
The significant change that occurred in ASK and ABILHAND-kids reveals that possible improvements in underlying components especially the active range of motion, reaction time, and strength of muscles, optimal muscle synergies, were integrated into daily activities. This result is in line with a recently published study by Şahin et al. They investigated the effects of 8 weeks of VR intervention on sixty children with HCP and reported that activities of daily living based on Wee Functional Independence Measure were improved [11]. Other studies have revealed that VR intervention promotes the functional tasks commonly used in daily activities such as reaching, stepping, standing, walking, running, and jumping [36,53]. Therefore, practicing functional tasks in a purposeful and playful context can result in skilled functional activities.
According to the MCID values, 62.25%, 75%, 87.5%, and 50% of participants demonstrated clinically significant changes in their PBS, TUG, BBT less-affected hand, and BBT more-affected hand scores, respectively. The multi-component VR program was designed to target functional motor deficits that are commonly experienced by children with HCP. By addressing these deficits, the intervention may have improved voluntary movements and consequently active participation in the majority of children with HCP.
The main distinguishable strength of this study was the use of a novel multi-component VR program that engaged all body segments during upper extremity tasks, static postural training, and dynamic balance training. Another strength of the present study was the use of a wide range of outcome measures to evaluate improvements in motor skills and functional independence after a short duration of the multi-component VR intervention in children with HCP. These results support the understanding that the inclusion of a diversity of motor tasks is probably the suitable method to obtain significant improvements in the short duration of intervention. However, there are some potential limitations. The first is selection bias. Due to our study design, observed changes may be limited to the study context or the enrolled participants, so our results may not be generalizable to the entire population. The second is the lack of a control group, which makes it impossible to compare the results with other approaches. The third is the absence of a follow-up phase, which leads to an inconclusive finding about the long-term sustainability of improvements. Therefore, the results of this study need to be confirmed by future RCTs.
5. Conclusions
The results of the present study provide early evidence for the effectiveness of multi-component VR-based programs as an efficient method to improve activities and participation in children with HCP. However, future RCT studies are needed to examine the long-term effects and compare the efficacy with conventional occupational therapy practice.
What this paper adds?
Virtual reality (VR) technologies are used commonly in rehabilitation clinical settings. Ongoing gaps in the implementation of VR technologies in neurorehabilitation provide an important opportunity to develop and test new VR systems with more capabilities. This study evaluates the feasibility of a new multi-component VR-based program on motor skills and functional postural control for children with hemiplegic cerebral palsy (HCP). This VR program addresses upper extremities, dynamic balance, and static postural control to improve children's functional motor skills. Both visual and statistical analyses were used to investigate the feasibility of using the VR-based program. The data of this single case experimental study show that the multi-component VR-based program is a promising modality to improve functional postural control, upper extremity functions, and independence in activities of daily living in children with HCP. This study suggests that the multi-component VR program is a feasible and effective approach for children with HCP.
Author contribution statement
Meysam Roostaei: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Marzieh Babaee; Saeed Behzadipour: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Seyedmostafa Alavian; Narjes Jafari; Seyed Mansoor Rayegani: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e19883.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Roostaei M., et al. The relationship between functional motor Status and self-evaluation in individuals with cerebral palsy: a systematic review. Iran. J. Child Neurol. 2020;15(3) doi: 10.22037/ijcn.v15i4.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallman-Cooper J.L., Rocha Cabrero F. StatPearls Publishing; Treasure Island (FL: 2022. Cerebral Palsy. [PubMed] [Google Scholar]
- 3.Jonsson U., et al. Cerebral palsy prevalence, subtypes, and associated impairments: a population‐based comparison study of adults and children. Dev. Med. Child Neurol. 2019;61(10):1162–1167. doi: 10.1111/dmcn.14229. [DOI] [PubMed] [Google Scholar]
- 4.Roostaei M., et al. Developmental Neurorehabilitation; 2021. Effect of Upper Extremity Constraints on Functional and Dynamic Postural Control in Children with Hemiplegic Cerebral Palsy; pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 5.Saygı E.K. Hand Function. Springer; 2019. Hand function in cerebral palsy; pp. 181–188. [Google Scholar]
- 6.Pashmdarfard M., Richards L.G., Amini M. Factors affecting participation of children with cerebral palsy in meaningful activities: systematic review. Occup. Ther. Health Care. 2021;35(4):442–479. doi: 10.1080/07380577.2021.1938339. [DOI] [PubMed] [Google Scholar]
- 7.Seyed Mansoor R., Marzieh B., Seyed Ahmad R. In: Neurostimulation and Neuromodulation in Contemporary Therapeutic Practice. Denis L., Seyed Mansoor R., editors. 2020. Rehabilitation medicine management of Spasticity. (IntechOpen: Rijeka). Ch. 5. [Google Scholar]
- 8.Novak I., Honan I. Effectiveness of paediatric occupational therapy for children with disabilities: a systematic review. Aust. Occup. Ther. J. 2019;66(3):258–273. doi: 10.1111/1440-1630.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackman M., et al. Interventions to improve physical function for children and young people with cerebral palsy: international clinical practice guideline. Dev. Med. Child Neurol. 2022;64(5):536–549. doi: 10.1111/dmcn.15055. [DOI] [PubMed] [Google Scholar]
- 10.Balcı N.Ç. Cerebral Palsy-Current Steps. IntechOpen; 2016. Current rehabilitation methods for cerebral palsy. [Google Scholar]
- 11.Şahin S., et al. The effects of virtual reality on motor functions and daily life activities in unilateral spastic cerebral palsy: a single-blind randomized controlled trial. Game. Health J. 2020;9(1):45–52. doi: 10.1089/g4h.2019.0020. [DOI] [PubMed] [Google Scholar]
- 12.Menekseoglu A.K., et al. Effect of a irtual reality-mediated gamified rehabilitation program on upper limb functions in children with hemiplegic cerebral palsy: a prospective, randomized controlled study. Am. J. Phys. Med. Rehabil. 2023;102(3):198–205. doi: 10.1097/PHM.0000000000002060. [DOI] [PubMed] [Google Scholar]
- 13.Atasavun Uysal S., Baltaci G. Effects of nintendo Wii(™) training on occupational performance, balance, and daily living activities in children with spastic hemiplegic cerebral palsy: a single-blind and randomized trial. Game. Health J. 2016;5(5):311–317. doi: 10.1089/g4h.2015.0102. [DOI] [PubMed] [Google Scholar]
- 14.Demers M., Fung K. Integration of Motor Learning Principles Into Virtual Reality Interventions for Individuals With Cerebral Palsy: Syst. Rev. 2021;9(2) doi: 10.2196/23822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elnaggar R.K., et al. Effectiveness of a multi-modal exercise program incorporating plyometric and balance training in children with hemiplegic cerebral palsy: a three-armed randomized clinical trial. Phys. Occup. Ther. Pediatr. 2022;42(2):113–129. doi: 10.1080/01942638.2021.1964674. [DOI] [PubMed] [Google Scholar]
- 16.Maitre N.L., et al. Kinematic and somatosensory gains in infants with cerebral palsy after a multi-component upper-extremity intervention: a randomized controlled trial. Brain Topogr. 2020;33:751–766. doi: 10.1007/s10548-020-00790-5. [DOI] [PubMed] [Google Scholar]
- 17.Organization W.H. World Health Organization; 2007. International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY. [Google Scholar]
- 18.Rathinam C., et al. Effectiveness of virtual reality in the treatment of hand function in children with cerebral palsy: a systematic review. J. Hand Ther. 2019;32(4):426–434.e1. doi: 10.1016/j.jht.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Abdelhaleem N., El Wahab M.S.A., Elshennawy S. Effect of virtual reality on motor coordination in children with cerebral palsy: a systematic review and meta-analysis of randomized controlled trials. Egyptian Journal of Medical Human Genetics. 2022;23(1):71. [Google Scholar]
- 20.Krasny-Pacini A., Evans J. Single-case experimental designs to assess intervention effectiveness in rehabilitation: a practical guide. Annals of Physical and Rehabilitation Medicine. 2018;61(3):164–179. doi: 10.1016/j.rehab.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Hashemi Y., et al. The effects of supervised and non-supervised upper limb virtual reality exercises on upper limb sensory-motor functions in patients with idiopathic Parkinson's disease. Hum. Mov. Sci. 2022;85 doi: 10.1016/j.humov.2022.102977. [DOI] [PubMed] [Google Scholar]
- 22.Johnson C., et al. Psychometric properties of functional postural control tests in children: a systematic review. Annals of Physical and Rehabilitation Medicine. 2023;66(4) doi: 10.1016/j.rehab.2022.101729. [DOI] [PubMed] [Google Scholar]
- 23.Gan S.-M., et al. Psychometric properties of functional balance assessment in children with cerebral palsy. Neurorehabilitation Neural Repair. 2008;22(6):745–753. doi: 10.1177/1545968308316474. [DOI] [PubMed] [Google Scholar]
- 24.Sanjivani N D., Prema A K. Intra-rater reliability of timed ‘up and go’test for children diagnosed with cerebral palsy. Int. J. Ther. Rehabil. 2012;19(10):575–580. [Google Scholar]
- 25.Hassani S., et al. O ne‐M inute W alk and modified T imed U p and G o tests in children with cerebral palsy: performance and minimum clinically important differences. Dev. Med. Child Neurol. 2014;56(5):482–489. doi: 10.1111/dmcn.12325. [DOI] [PubMed] [Google Scholar]
- 26.Alimi E., et al. Test-retest & inter-rater reliability of Persian version of pediatric balance scale in children with spastic cerebral palsy. Iran. J. Child Neurol. 2019;13(4):163. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C.-l., et al. Validity, responsiveness, minimal detectable change, and minimal clinically important change of Pediatric Balance Scale in children with cerebral palsy. Res. Dev. Disabil. 2013;34(3):916–922. doi: 10.1016/j.ridd.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Liang K.-J., et al. Measurement properties of the box and block test in children with unilateral cerebral palsy. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-00379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadkhani-Pordanjani E., et al. Validity and reliability of the Persian ABILHAND-Kids in a sample of Iranian children with cerebral palsy. Disabil. Rehabil. 2020;42(12):1744–1752. doi: 10.1080/09638288.2018.1530307. [DOI] [PubMed] [Google Scholar]
- 30.Dehghan S.K., et al. Validity and reliability of Activities Scale for Kids (ASK) in children with cerebral palsy. Journal of Research in Rehabilitation Sciences. 2011;7(3) [Google Scholar]
- 31.Begun A. SWK 3402 Online Coursebook: Research & Statistics for Understanding Social Work Interventions. The Ohio State University; Open Educational Resources: 2018. Module 4 chapter 3: analysis of single system design data. [Google Scholar]
- 32.Brossart D.F., Laird V.C., Armstrong T.W. Interpreting Kendall's Tau and Tau-U for single-case experimental designs. Cogent Psychology. 2018;5(1) [Google Scholar]
- 33.Tarlow K.R. An improved rank correlation effect size statistic for single-case designs: baseline corrected Tau. Behav. Modif. 2017;41(4):427–467. doi: 10.1177/0145445516676750. [DOI] [PubMed] [Google Scholar]
- 34.Tarlow K. 2016. Baseline Corrected Tau Calculator.http://www.ktarlow.com/stats/tau Available from: [Google Scholar]
- 35.Dinomais M., et al. A new virtual reality tool for unilateral cerebral palsy rehabilitation: two single-case studies. Dev. Neurorehabil. 2013;16(6):418–422. doi: 10.3109/17518423.2013.778347. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y.-P., et al. Use of virtual reality to improve upper-extremity control in children with cerebral palsy: a single-subject design. Phys. Ther. 2007;87(11):1441–1457. doi: 10.2522/ptj.20060062. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima N., et al. The relationship between proximal function of the upper extremity on the paralyzed side and upper extremity skills in daily life of subacute stroke patients. Japanese Journal of Comprehensive Rehabilitation Science. 2017;8:44–50. [Google Scholar]
- 38.Amano Y., et al. Reaching exercise for chronic paretic upper extremity after stroke using a novel rehabilitation robot with arm-weight support and concomitant electrical stimulation and vibration: before-and-after feasibility trial. Biomed. Eng. Online. 2020;19(1):28. doi: 10.1186/s12938-020-00774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burn M.B., Gogola G.R. HAND; 2021. Dexterity of the Less Affected Hand in Children with Hemiplegic Cerebral Palsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rich T.L., et al. Less-affected hand function in children with hemiparetic unilateral cerebral palsy: a comparison study with typically developing peers. Neurorehabilitation Neural Repair. 2017;31(10–11):965–976. doi: 10.1177/1545968317739997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metzler M.J., et al. Feasibility of high repetition upper extremity rehabilitation for children with unilateral cerebral palsy. Phys. Occup. Ther. Pediatr. 2022;42(3):242–258. doi: 10.1080/01942638.2021.2010857. [DOI] [PubMed] [Google Scholar]
- 42.Shih T.-Y., et al. Comparative effects of kinect-based versus therapist-based constraint-induced movement therapy on motor control and daily motor function in children with unilateral cerebral palsy: a randomized control trial. J. NeuroEng. Rehabil. 2023;20(1):13. doi: 10.1186/s12984-023-01135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bleyenheuft Y., et al. Motor skill training may restore impaired corticospinal tract fibers in children with cerebral palsy. Neurorehabilitation Neural Repair. 2020;34(6):533–546. doi: 10.1177/1545968320918841. [DOI] [PubMed] [Google Scholar]
- 44.Adamovich S.V., et al. Sensorimotor training in virtual reality: a review. NeuroRehabilitation. 2009;25(1):29–44. doi: 10.3233/NRE-2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pourazar M., Bagherzadeh F., Mirakhori F. Virtual reality training improves dynamic balance in children with cerebral palsy. Int. J. Dev. Disabil. 2021;67(6):429–434. doi: 10.1080/20473869.2019.1679471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavão S.L., et al. Effect of the severity of manual impairment and hand dominance on anticipatory and compensatory postural adjustments during manual reaching in children with cerebral palsy. Res. Dev. Disabil. 2018;83:47–56. doi: 10.1016/j.ridd.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 47.dos Santos Soares L.M., Rozane J.M.S.G., de Paula Carvalho R. Motor performance of children with cerebral palsy in anterior reach. Clin. BioMech. 2019;68:158–162. doi: 10.1016/j.clinbiomech.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Galgon A.K., Shewokis P.A., Tucker C.A. Changes in standing postural control during acquisition of a sequential reaching task. Gait Posture. 2010;31(2):265–271. doi: 10.1016/j.gaitpost.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Tarakci D., et al. Wii-based balance therapy to improve balance function of children with cerebral palsy: a pilot study. J. Phys. Ther. Sci. 2013;25(9):1123–1127. doi: 10.1589/jpts.25.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westcott S.L., Burtner P. Postural control in children: implications for pediatric practice. Phys. Occup. Ther. Pediatr. 2004;24(1–2):5–55. doi: 10.1300/j006v24n01_02. [DOI] [PubMed] [Google Scholar]
- 51.Roostaei M., et al. The effect of dual-task conditions on gait and balance performance in children with cerebral palsy: a systematic review and meta-analysis of observational studies. J. Bodyw. Mov. Ther. 2021;26:448–462. doi: 10.1016/j.jbmt.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Elhinidi E.I.M., Ismaeel M.M.I., El-Saeed T.M. Effect of dual-task training on postural stability in children with infantile hemiparesis. J. Phys. Ther. Sci. 2016;28(3):875–880. doi: 10.1589/jpts.28.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnoni J.L.B., et al. Effects of virtual reality in body oscillation and motor performance of children with cerebral palsy: a preliminary randomized controlled clinical trial. Compl. Ther. Clin. Pract. 2019;35:189–194. doi: 10.1016/j.ctcp.2019.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.