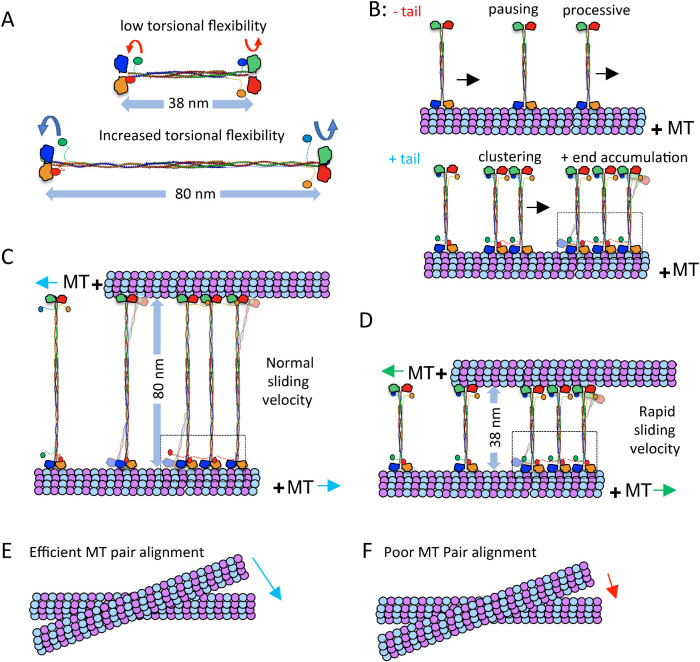
FIGURE 6:
Kinesin-5 MT sliding motility is tuned by the tail to motor mediated clustering and the length of the bipolar minifilament. A) Kinesin-5 mini-tetramers are 38 nm in length while native kinesin-5 motors are 60–80 nm in length. The decreased minifilament length leads to a decrease in torsional flexibility in the minitetramers, compared with the high-torsional flexibility of native kinesin-5. B) Top panel, kinesin-5 minitetramers, without the tail domain, show processive motility punctuated by pauses and short residence time at MT plus-ends. The bottom panel, kinesin-5 minitetramers, with the tail domain, show increased pausing, coupled with motor clustering mediated by cross motor-tail interactions between multiple minitetramers. C) MT sliding motility mediated by native kinesin-5 leads to 80-nm separation between paired MTs, which slide apart with normal sliding velocity, which is punctuated by pauses and is lower than twice the velocity of each motor ends along each MT. D) MT sliding motility by the kinesin-5 minitetramers leads to 38-nm separation between the paired MTs and a more efficient MT sliding motility that approaches closely to twice the velocity of each motor end. E) The native kinesin-5 motor MT pair alignment is efficient due to the torsional flexibility of the minifilament F) Kinesin-5 mini-tetramer MT pair alignment is poor due to decreased torsional flexibility of its shortened minifilament.