Abstract
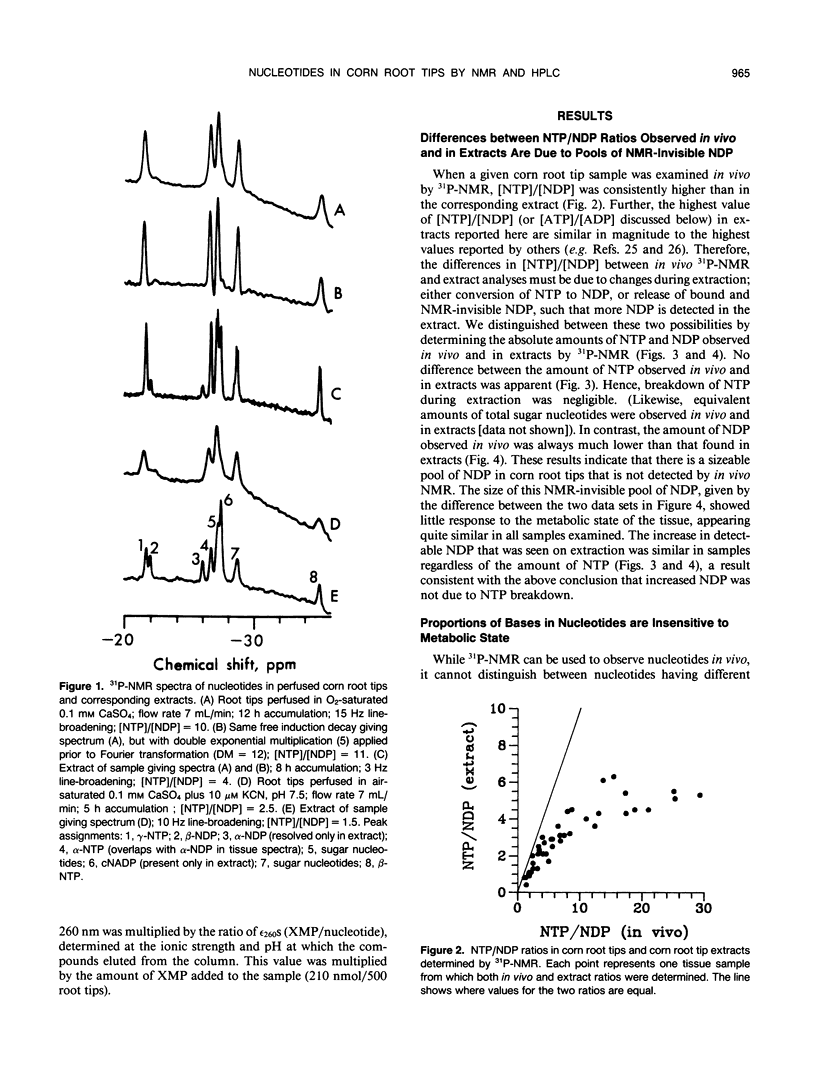
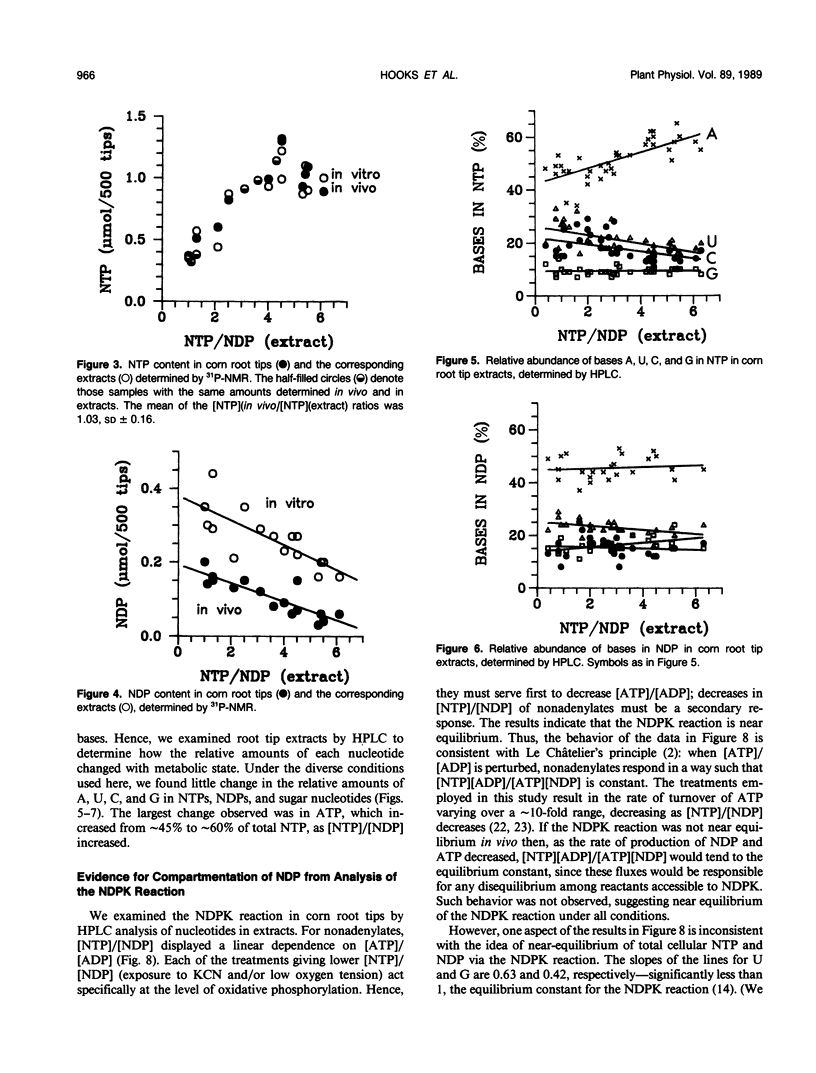
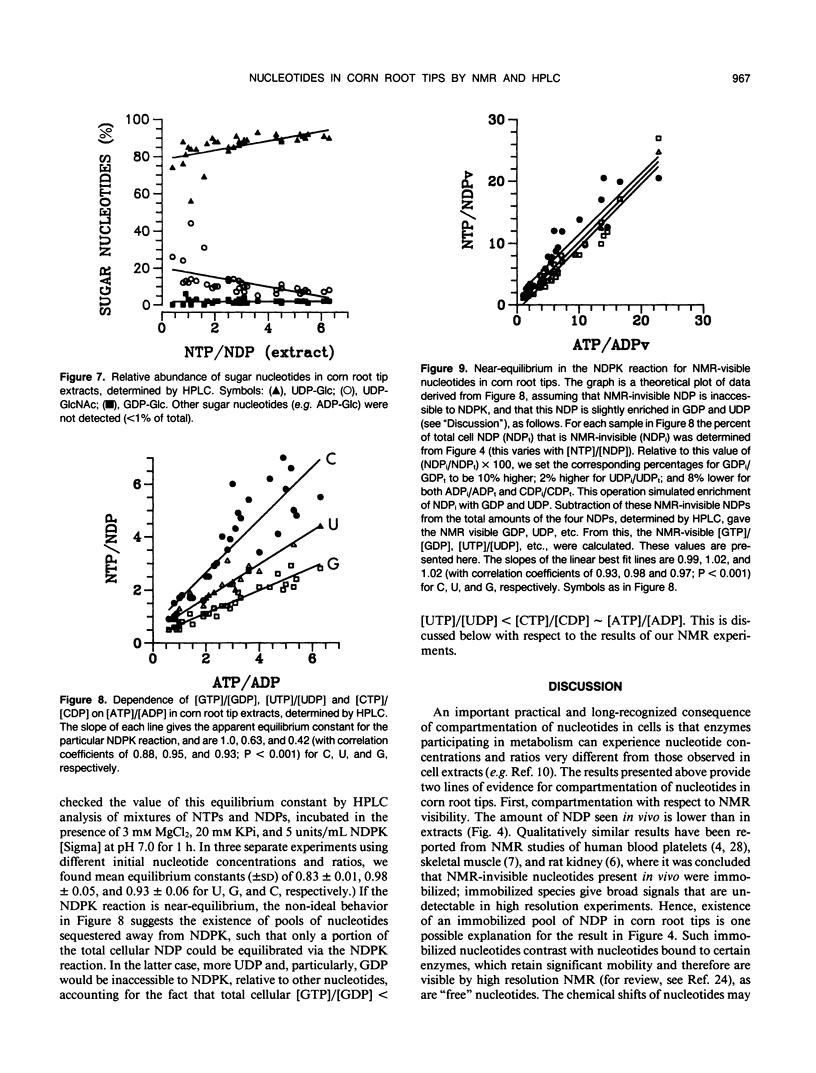
Corn (Zea mays L.) root tips were subjected to different conditions so that nucleotide levels varied over a wide range. Levels of nucleotides in corn root tips were measured using 31P nuclear magnetic resonance (NMR) spectroscopy and high performance liquid chromatography. Results indicate: (a) Similar amounts of NTP and sugar nucleotides were observed by in vivo NMR and in extracts. In contrast, a significant amount of NDP observed in root tip extracts was not detected by in vivo NMR. Thus, for a given sample, [NTP]/[NDP] ratios determined in vivo by 31P-NMR are always higher than ratios observed in extracts, deviating by ∼4-fold at the highest ratios. The NMR-invisible pool of NDP appeared quite metabolically inert, barely changing in size as total cell NDP changed. We conclude that NDP in corn root tips is compartmented with respect to NMR visibility, and that it is the NMR-visible pool which responds dynamically to metabolic state. The NMR-invisible NDP could either be immobilized (and so have broad, undetectable NMR signals), or be complexed with species that cause the chemical shift of NDP to change (so it does not contribute to the NMR signal of free NDP), or both. (b) 31P-NMR cannot distinguish between bases (A, U, C, and G) of nucleotides. HPLC analysis of root tip extracts showed that the relative amount of each base in the NTP and NDP pools was quite constant in the different samples. (c) In extracts, for each of the nonadenylate nucleotides, [NTP]/[NDP] was linearly proportional to [ATP]/[ADP], indicating near equilibrium in the nucleoside diphosphokinase (NDPK) reaction. However, the apparent equilibrium constants for the phosphorylation of GDP and UDP by ATP were significantly lower than 1, the true equilibrium constant for the NDPK reaction. Thus, for a given sample, [ATP]/[ADP] ∼ [CTP]/[CDP] > [UTP]/[UDP] > [GTP]/[GDP]. This result suggests that the different NDPs in corn root tips do not have equal access to NDPK.
Full text
PDF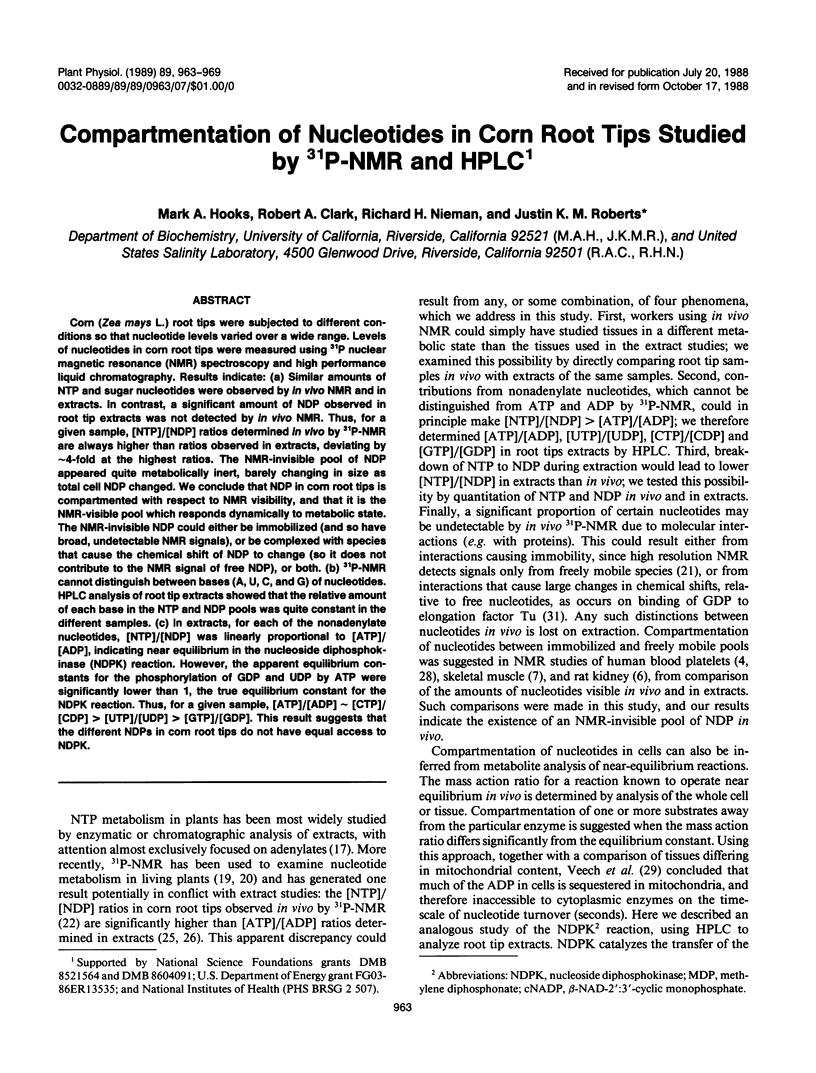



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Baki A. A., Ray P. M. Regulation by auxin of carbohydrate metabolism involved in cell wall synthesis by pea stem tissue. Plant Physiol. 1971 Apr;47(4):537–544. doi: 10.1104/pp.47.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. L., Dobson C. M., Kirk K. L., Poulsen F. M., Valeri C. R., Vecchione J. J. Studies of human platelets by 19F and 31P NMR. FEBS Lett. 1979 Mar 1;99(1):141–146. doi: 10.1016/0014-5793(79)80266-5. [DOI] [PubMed] [Google Scholar]
- Freeman D., Bartlett S., Radda G., Ross B. Energetics of sodium transport in the kidney. Saturation transfer 31P-NMR. Biochim Biophys Acta. 1983 Apr 5;762(2):325–336. doi: 10.1016/0167-4889(83)90087-3. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Smith H., Taylor E. W. Tublin: nucleotide binding and enzymic activity. J Mol Biol. 1974 Nov 5;89(3):455–468. doi: 10.1016/0022-2836(74)90475-6. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Pyridine nucleotides and rate control. Symp Soc Exp Biol. 1973;27:299–318. [PubMed] [Google Scholar]
- Ogawa S., Lee T. M. Proton stoichiometry of adenosine 5'-triphosphate synthesis in rat liver mitochondria studied by phosphorus-31 nuclear magnetic resonance. Biochemistry. 1982 Aug 31;21(18):4467–4473. doi: 10.1021/bi00261a042. [DOI] [PubMed] [Google Scholar]
- Rao B. D., Cohn M. 31P nuclear magnetic resonance of bound substrates of arginine kinase reaction: chemical shifts in binary, ternary, quaternary, and transition state analog complexes. J Biol Chem. 1977 May 25;252(10):3344–3350. [PubMed] [Google Scholar]
- Roberts J. K., Lane A. N., Clark R. A., Nieman R. H. Relationships between the rate of synthesis of ATP and the concentrations of reactants and products of ATP hydrolysis in maize root tips, determined by 31P nuclear magnetic resonance. Arch Biochem Biophys. 1985 Aug 1;240(2):712–722. doi: 10.1016/0003-9861(85)90080-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Wemmer D., Jardetzky O. Measurement of mitochondrial ATPase activity in maize root tips by saturation transfer p nuclear magnetic resonance. Plant Physiol. 1984 Mar;74(3):632–639. doi: 10.1104/pp.74.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Drew M. C., Pradet A. Metabolic Acclimation to Anoxia Induced by Low (2-4 kPa Partial Pressure) Oxygen Pretreatment (Hypoxia) in Root Tips of Zea mays. Plant Physiol. 1988 Jan;86(1):61–66. doi: 10.1104/pp.86.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Raymond P., Pradet A. Metabolic Activity and Energy Charge of Excised Maize Root Tips under Anoxia: CONTROL BY SOLUBLE SUGARS. Plant Physiol. 1980 Dec;66(6):1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurbil K., Holmsen H., Shulman R. G. Adenine nucleotide storage and secretion in platelets as studied by 31P nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1979 May;76(5):2227–2231. doi: 10.1073/pnas.76.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Wilson A. M., Harris G. A. Hexose-, inositol-, and nucleoside phosphate esters in germinating seeds of crested wheatgrass. Plant Physiol. 1966 Nov;41(9):1416–1419. doi: 10.1104/pp.41.9.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittinghofer A., Goody R. S., Roesch P., Kalbitzer H. R. The structure of the EF-Tu . GDP . Me2+ complex. Eur J Biochem. 1982 May;124(1):109–115. doi: 10.1111/j.1432-1033.1982.tb05912.x. [DOI] [PubMed] [Google Scholar]


