Figure 1. Screening, Randomization, and Follow-up.
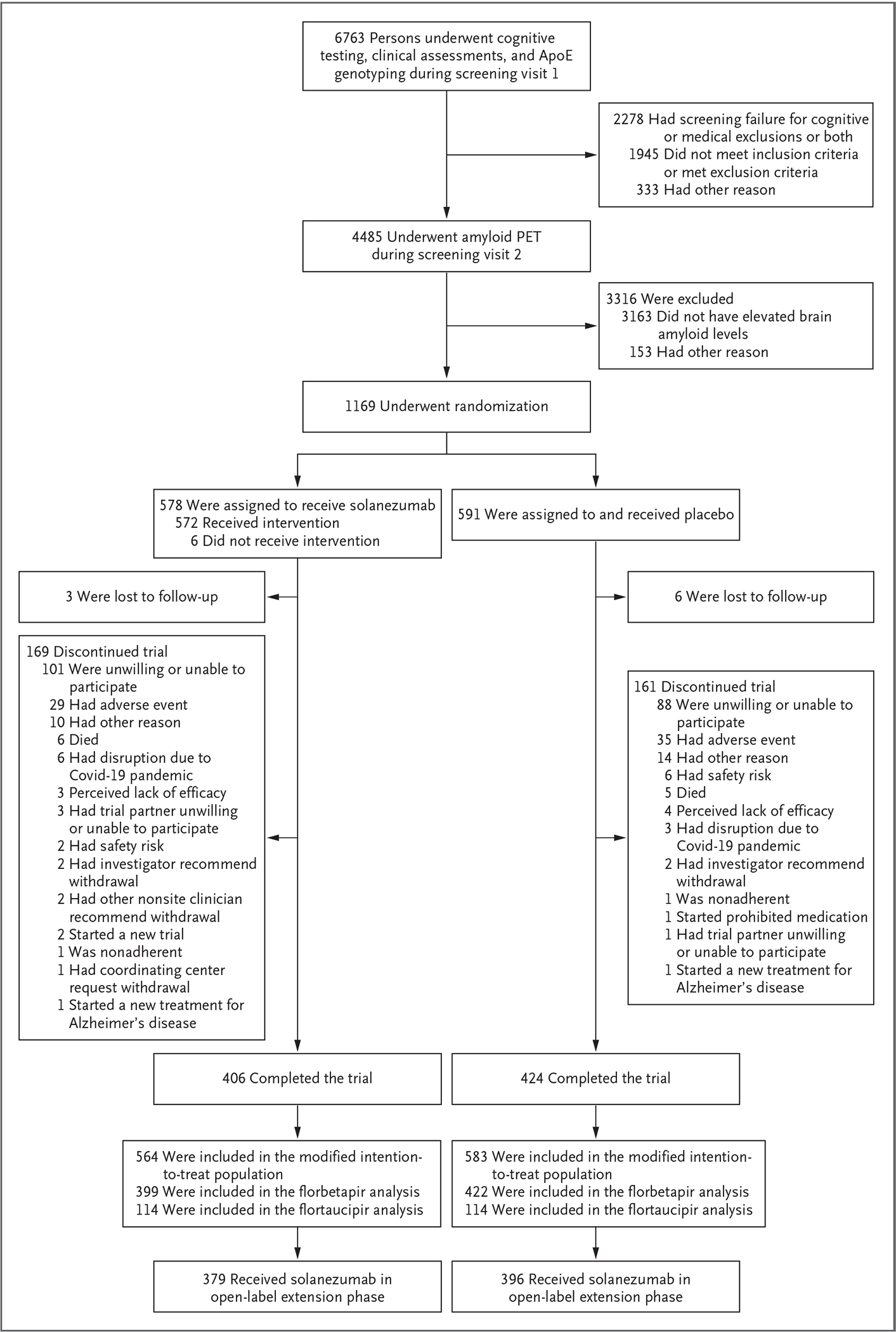
The modified intention-to-treat population included randomly assigned participants who received at least one dose of solanezumab or placebo and underwent assessment for the primary end point. Participants who completed visit 66 (target, 240 weeks) were considered to have completed the double-blind phase. ApoE denotes apolipoprotein E, Covid-19 coronavirus disease 2019, and PET positron-emission tomography.
