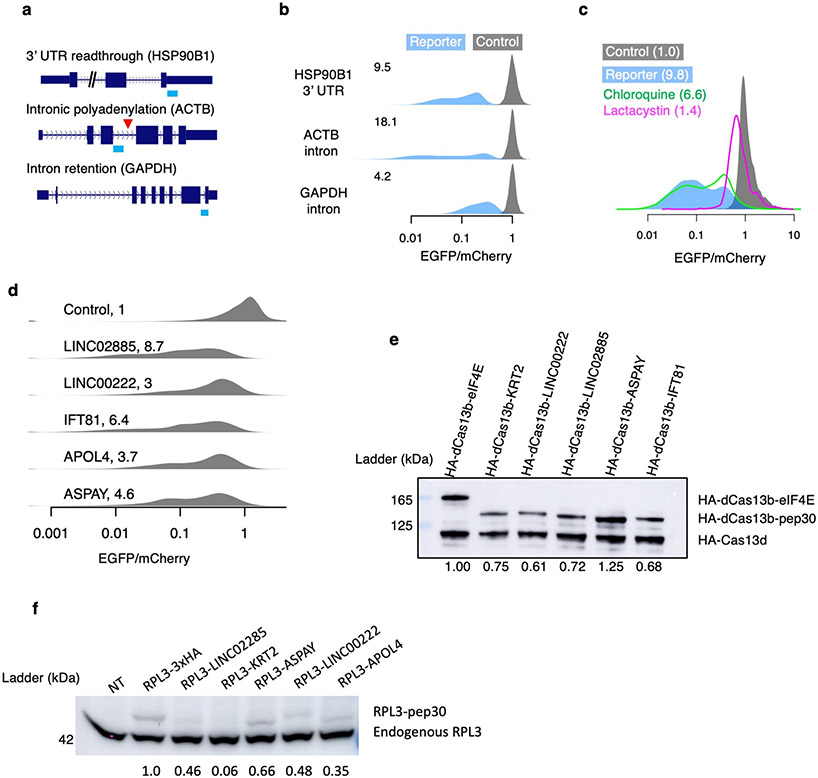
Extended Data Fig. 1 ∣. Translation surveillance of representative noncoding sequences.
a, Noncoding sequences in the HSP90B1 3’ UTR, an ACTB intron, and a GAPDH intron were cloned into the bicistronic reporter system shown in Fig. 1b. b, Density plots for the distribution of EGFP/mCherry ratios as measured by flow cytometry 24 hours after reporter transfection. The median fold loss of EGFP/mCherry ratio relative to control is shown on the top left corner of each density plot. c, Density plot of the EGFP/mCherry ratio for cells transfected with either the control or the ACTB intron reporter, alone or with simultaneous treatment of either proteasome inhibitor (lactacystin) or lysosome inhibitor (chloroquine). The numbers indicate the median fold loss of EGFP/mCherry relative to control. d-f, six noncoding sequences from the Pep30 library (KRT2 intron, APOL4 intron, LINC00222, LINC02885, ASPAY 3’ UTR, and IFT81 3’ UTR) were selected and cloned into either the original mCherry-EGFP bicistronic reporter (d, cloning failed for KRT2), fused to the C-terminus of HA-tagged PspCas13b protein (e, cloning failed for APOL4), or fused to the C-terminus of RPL3 (f, cloning failed for IFT81). d, Same as b for indicated noncoding sequences. e, Equal amount of HA-dPspCas13b-pep30 reporter plasmids were co-transfected with a HA-RfxCas13d plasmid and the protein abundance was assayed by western blotting with an HA antibody. HA-dCas13b fused to human protein eIF4E was used as a control. The abundance of HA-dCas13b-pep30 was quantified by first normalizing to HA-Cas13d then to eIF4E fusion. f, Equal amount of RPL3 reporter plasmids were transfected into HEK293T cells and western blots were performed using an RPL3 antibody, which detects both endogenous RPL3 (lower bands) and the RPL3 reporter protein (upper bands). NT: no transfection control. The level of the reporter protein was first normalized to endogenous RPL3 and then to the RPL3-3xHA sample. N=4 biological replicates.