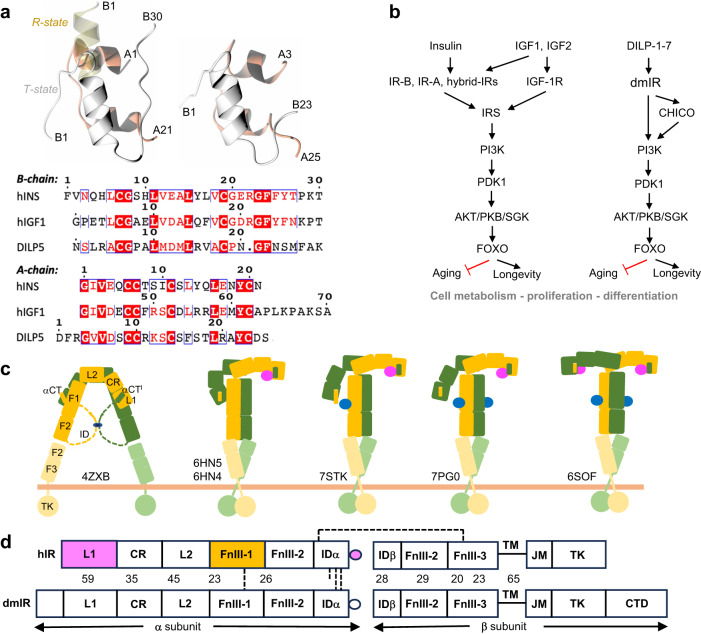
Fig. 1. Summary of the conservation of IIS axis in Drosophila and humans.
a Structural similarity of human insulin (top left) and DILP5 (right), B-chains in grey, A-chains in coral, pale yellow—insulin’s B1-B6 N-terminus in the so-called R-state; bottom – the sequence alignment of human insulin, hIGF-1 (its C-domain is omitted here as not relevant for DILPs) and DILP5. b Functional homology and conservation of IIS axis in humans and Drosophila, with some representatives of the key and common downstream effector proteins. c Schematic representation of the domain organisation, and examples of some key structural conformers, of the hIR in the apo (far left) and its holo forms; yellow/green—hIR protomers, insulins in site 1/1’ in pink, insulins in site 2/2’ in blue; PDB IDs are given for some representative structures. d Comparison of the domain organisation of hIR and dmIR αβ protomers (based on the sequence alignment), and their sequence similarity (in %); in magenta—domains involved in insulin binding site 1/1’, in yellow—site 2/2’; dashed lines - disulfide bonds; dmIR has only two inter-ID domains -SS- bonds, as it does not have the equivalent of human Cys682 in this region.