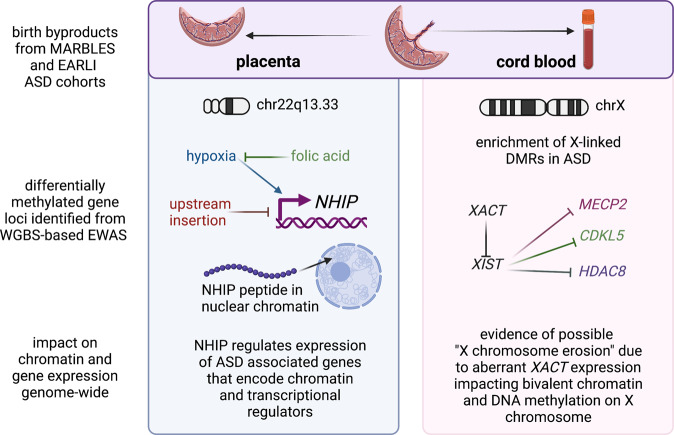
Fig. 2. Mechanistic insights from epigenomic signatures of idiopathic ASD newborn surrogate tissues.
At birth, the fetal derived byproducts of placenta and cord blood are usually discarded, but were collected as part of the prospective MARBLES and EARLI cohorts of enriched idiopathic ASD risk. Since they are derived from different lineages of the early embryo, placenta and cord blood from the same individuals have each revealed distinct mechanisms from their epigenomic signatures. Analyses of placenta (left panel) resulted in the discovery of the neuronal hypoxia inducible, placenta associated (NHIP) locus on 22q13.33. NHIP methylation levels were associated with both genetics (upstream insertion) and folic acid (prenatal vitamin use in first pregnancy month). In differentiated human neurons, hypoxia and resulting oxidative stress increase NHIP levels. ASD placenta and brain samples show significantly lower NHIP levels, suggesting a protective effect. NHIP is primate-specific and encodes a conserved micropeptide that associates with nuclear chromatin. NHIP elevated transcript levels alter many downstream gene targets enriched for regulators of chromatin and ASD genetic risk. In contrast, the idiopathic ASD epigenomic signature from cord blood samples revealed an enrichment for X-linked differentially methylated genes, as well as those involved in early neurodevelopment. Differential methylation was observed at the XACT locus, a primate-specific noncoding RNA expressed exclusively from the active X chromosome that represses XIST during the establishment of X chromosome inactivation. In human pluripotent stem cell culture, XACT is implicated in the phenomenon of X chromosome erosion, characterized by the partial loss of epigenetic silencing of X-linked genes and regions of bivalent chromatin on the X chromosome in females. While many X linked genes implicated in genetic risk for ASD were observed to be differentially methylated in ASD cord blood DNA, three specific examples (MECP2, CDKL5, and HDAC8) involved in syndromic forms of ASD are shown. Created with BioRender.com.