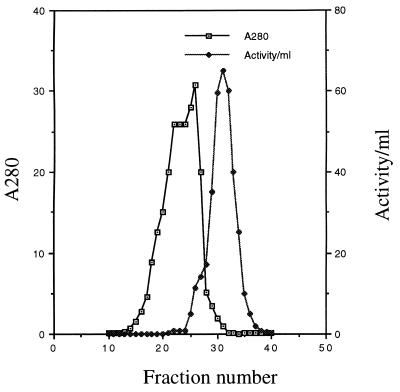
FIG. 1.
Partial purification of β-lactamase from M. tuberculosis H37Ra by gel filtration chromatography. Ammonium sulfate-precipitated protein from 5 liters of culture supernatant was suspended in 0.1 M sodium citrate and was loaded onto a G-75 Sephadex column. The eluted protein was collected in 10-ml fractions. The A280 value and the β-lactamase activity of each fraction are plotted. The large protein peak occurring before the peak of β-lactamase activity primarily comprises the bovine serum albumin added as a supplement to the broth culture.