Figure 7. High fucosylation is characteristic of proteins at the SV and plasma membrane.
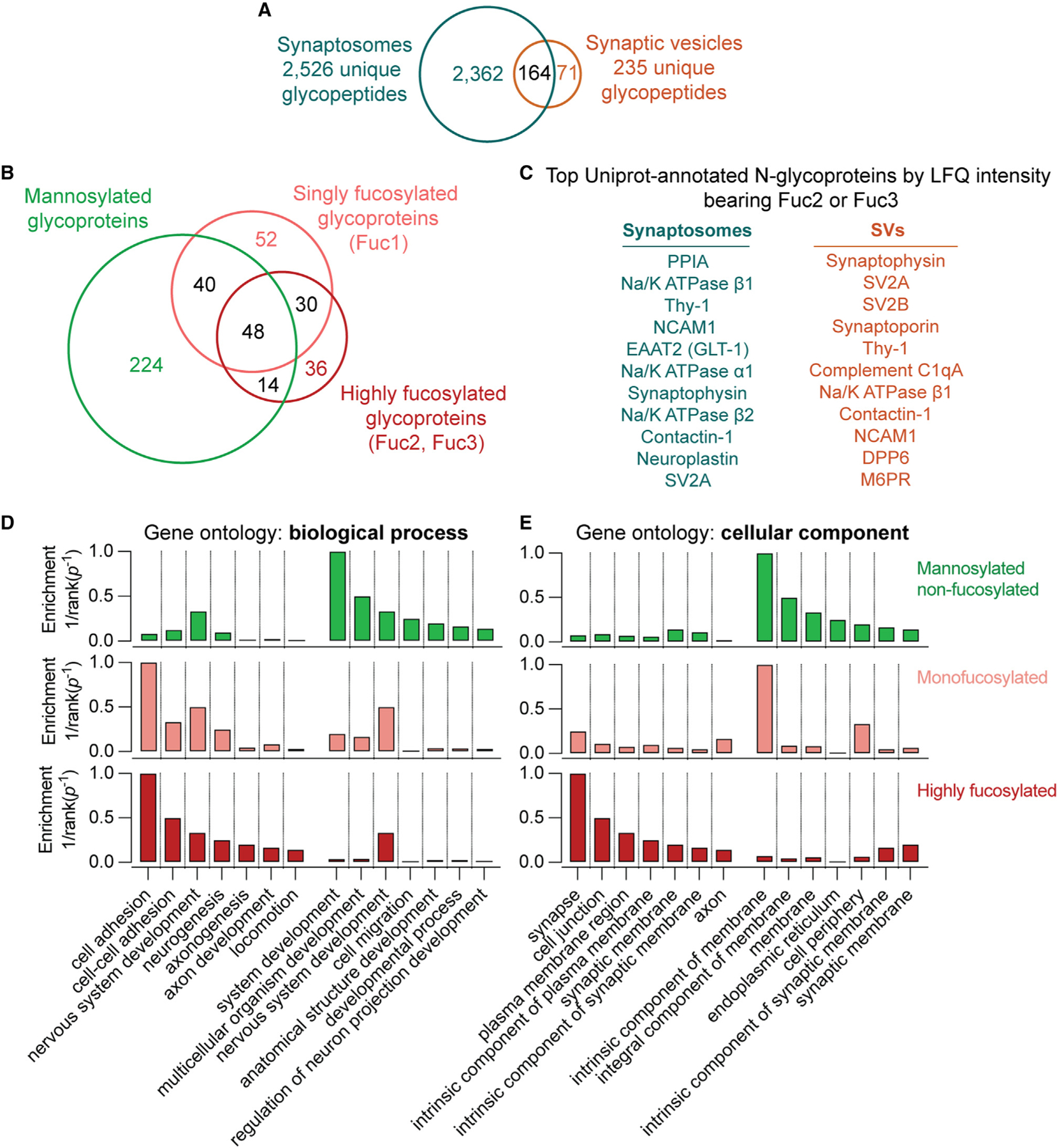
(A) Venn diagram of unique glycopeptides detected in synaptosomes and SVs. Approximately 70% of SV glycopeptides were also detected in synaptosome samples.
(B) Venn diagram of glycoproteins bearing N-glycans detected in this study. While some proteins were found with only one subtype of N-glycosylation, many proteins contained both mannosylated and fucosylated N-glycans.
(C) The top 11 proteins by LFQ intensity with Uniprot-annotated N-glycosylation sites in synaptosomes and SVs bearing antennary fucose, as defined by the presence of at least 2 fucoses. Antennary fucosylation was observed on almost all of the most abundant SV glycoproteins along with a number of cell adhesion proteins with roles in synaptic development.
(D) Enrichment for Gene Ontology (GO) biological process terms in non-fucosylated, singly fucosylated, and highly fucosylated proteins. GO terms were ranked by inverse p value for enrichment, and the top 7 terms enriched in highly fucosylated or non-fucosylated proteins are shown. Non-fucosylated proteins demonstrate substantially less enrichment of cell adhesion processes.
(E) As in (D) but for GO cellular component terms. Synapse- and plasma membrane-related components predominate in highly fucosylated proteins, while non-fucosylated proteins are more enriched in endoplasmic reticulum and non-specific membrane components.
See also Table S4, which contains all glycoprotein glycosylation category assignments and GO biological process enrichment data used to generate this figure.
