Abstract
Photosynthetic characteristics were studied in several F1 hybrids between C4 and C3-C4 species of Flaveria. Stable carbon isotope ratios, O2 inhibition of apparent photosynthesis, and phosphoenolpyruvate carboxylase activities in the hybrids were similar to the means for the parents. Values of CO2 compensation concentrations were nearer to those of the C4 parent and apparent photosynthesis was below that of both parents, being only 60 and 74% of that of the lowest (C3-C4) parent in two experiments. Reductions of CO2 compensation concentration and O2 inhibition of apparent photosynthesis as well as increases in carbon isotope ratios and phosphoenolpyruvate carboxylase activities compared to values in C3-C4 species suggest transfer of a limited degree of C4 photosynthesis to the F1 hybrids. However, the lower apparent photosynthesis of the hybrids suggests that transfer of C4 characteristics to non-C4 species is detrimental unless characteristics associated with C4 photosynthesis are fully developed. There was a highly significant negative correlation (r = −0.90) between CO2 compensation concentration and the logarithm of phosphoenolpyruvate carboxylase activity in the parents and hybrids, suggesting involvement of this enzyme in controlling the CO2 compensation concentration. Although bundle-sheath cells were more developed in leaves of hybrids than in C3-C4 parents, they appeared to contain lower quantities of organelles than those of the C4 parent. Reduced quantities of organelles in bundle-sheath cells could indicate incomplete compartmentation of partial pathways of the C4 cycle in the hybrids. This may mean that the reduction of CO2 compensation and O2 inhibition of apparent photosynthesis relative to the C3-C4 parents is less dependent on fully developed Kranz anatomy than is increased apparent photosynthesis.
Full text
PDF


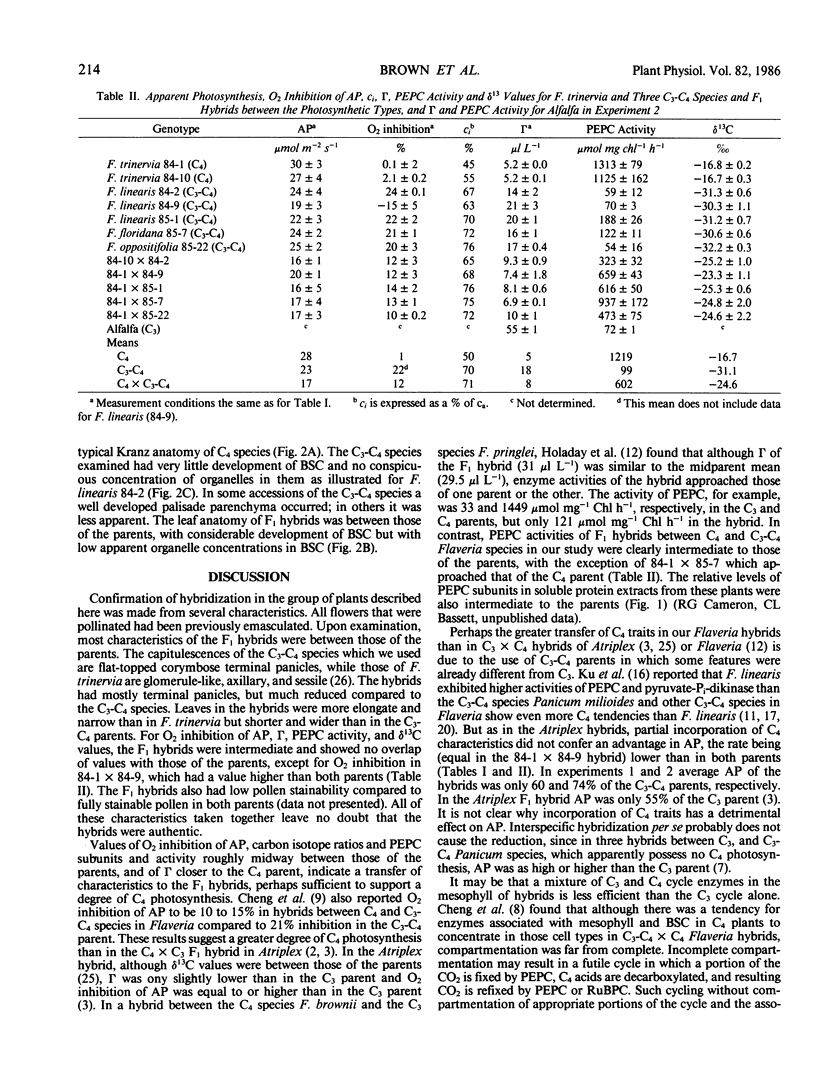
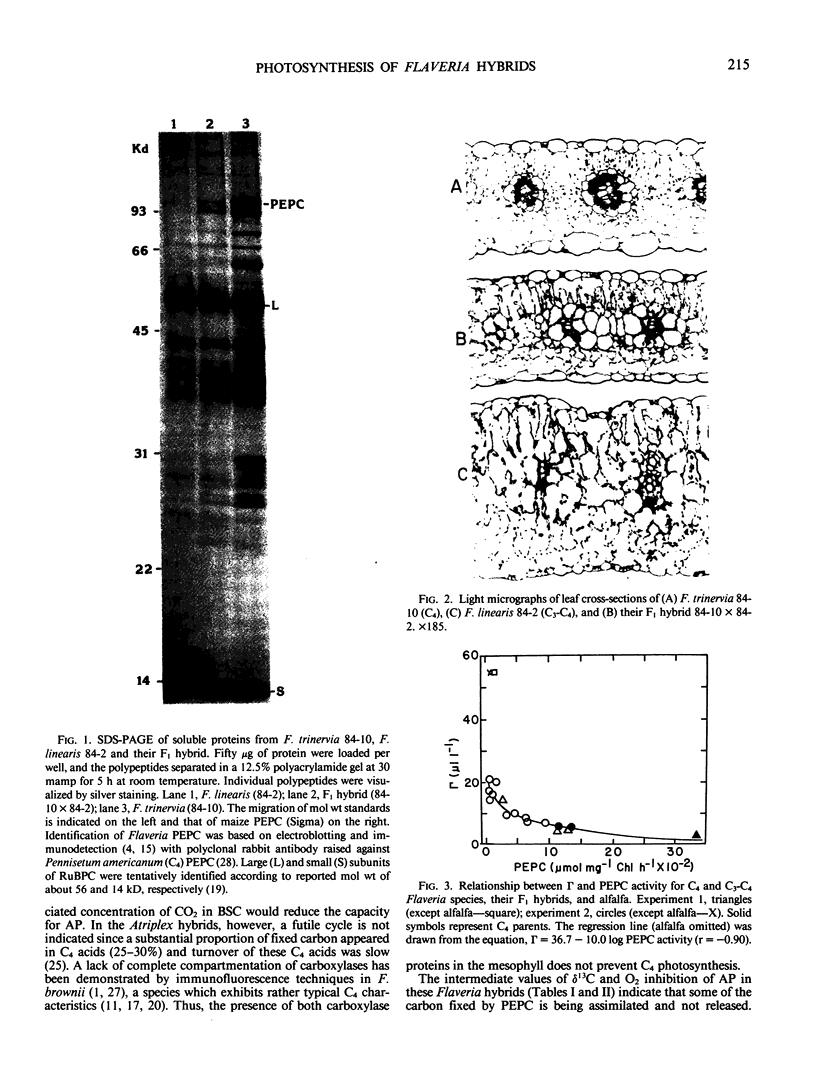


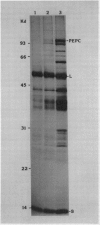
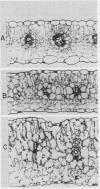
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Bouton J. H., Evans P. T., Malter H. E., Rigsby L. L. Photosynthesis, Morphology, Leaf Anatomy, and Cytogenetics of Hybrids between C(3) and C(3)/C(4)Panicum Species. Plant Physiol. 1985 Mar;77(3):653–658. doi: 10.1104/pp.77.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. H., Bouton J. H., Evans P. T. Oxygen Stimulation of Apparent Photosynthesis in Flaveria linearis. Plant Physiol. 1986 May;81(1):212–215. doi: 10.1104/pp.81.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskeep W. P., Bloom P. R. Extinction coefficients of chlorophyll a and B in n,n-dimethylformamide and 80% acetone. Plant Physiol. 1985 Feb;77(2):483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler D. P., Mayne B. C., Ray T. B., Goldstein L. D., Brown R. H., Black C. C. Biochemical components of the photosynthetic CO2 compensation point of higher plants. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1439–1446. doi: 10.1016/0006-291x(75)90520-3. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Visualization of antigenic proteins on Western blots. Anal Biochem. 1984 Jan;136(1):180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- Ku M. S., Monson R. K., Littlejohn R. O., Nakamoto H., Fisher D. B., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : I. Leaf Anatomy, Photosynthetic Responses to O(2) and CO(2), and Activities of Key Enzymes in the C(3) and C(4) Pathways. Plant Physiol. 1983 Apr;71(4):944–948. doi: 10.1104/pp.71.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moran R., Porath D. Chlorophyll determination in intact tissues using n,n-dimethylformamide. Plant Physiol. 1980 Mar;65(3):478–479. doi: 10.1104/pp.65.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A., Brown R. H. Photosynthesis in Grass Species Differing in Carbon Dioxide Fixation Pathways: II. A Search for Species with Intermediate Gas Exchange and Anatomical Characteristics. Plant Physiol. 1979 Aug;64(2):257–262. doi: 10.1104/pp.64.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Sternberg L. da S., Deniro M. J., Sloan M. E., Black C. C. Compensation point and isotopic characteristics of c(3)/c(4) intermediates and hybrids in panicum. Plant Physiol. 1986 Jan;80(1):242–245. doi: 10.1104/pp.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]