Abstract
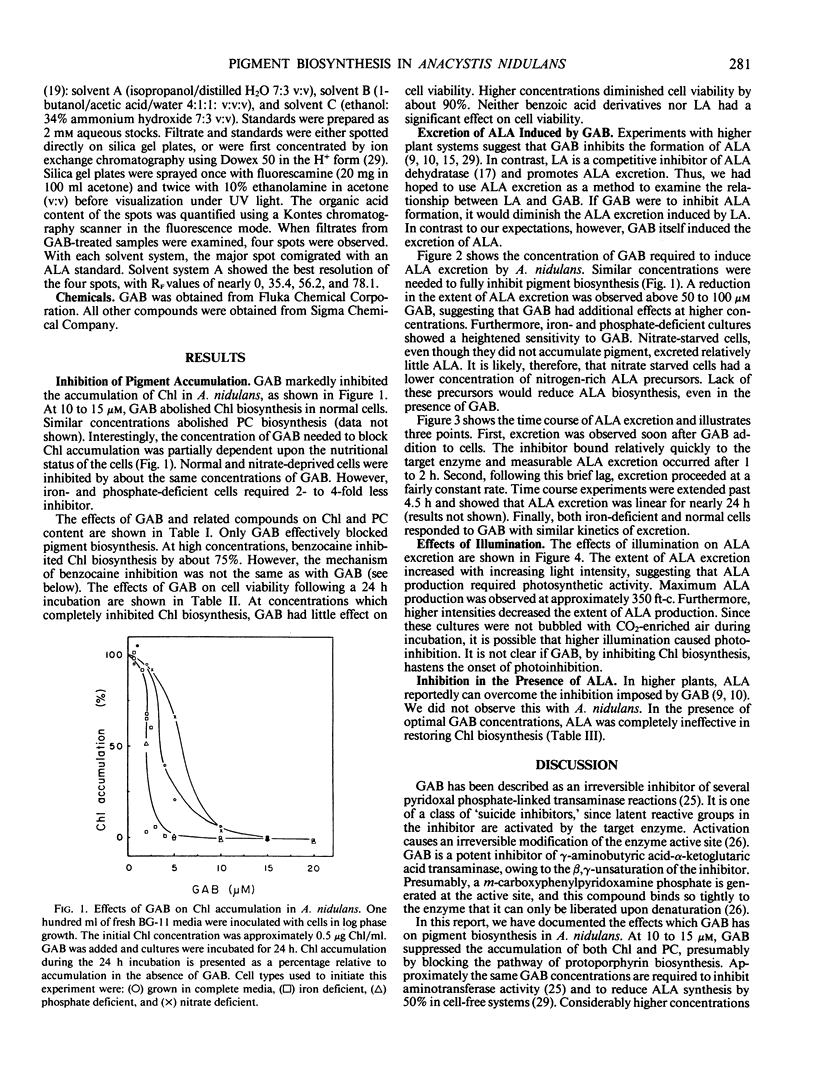
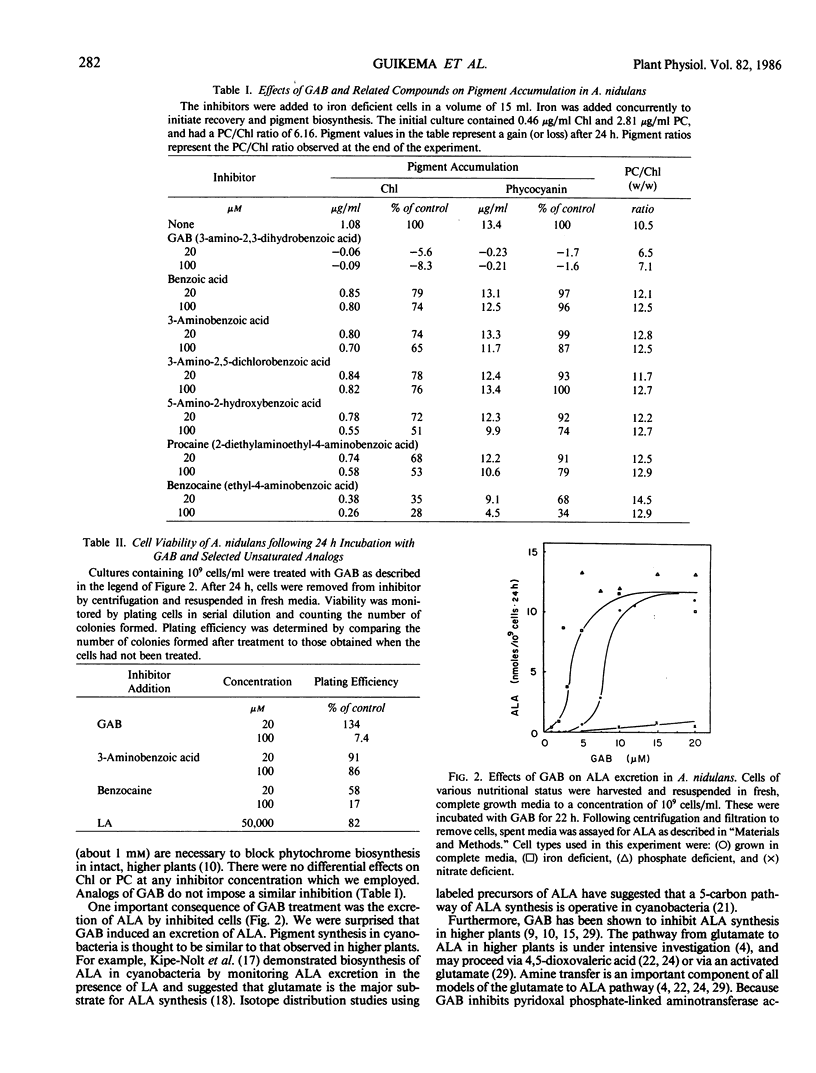
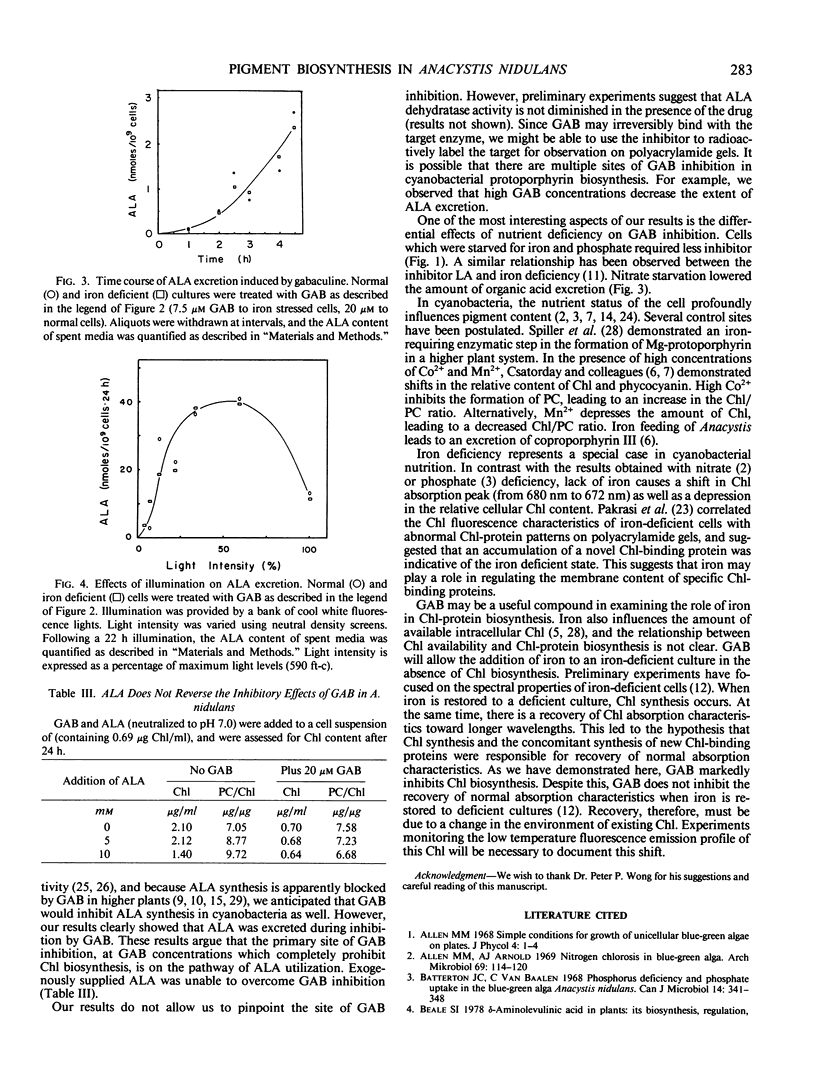
Pigment biosynthesis in the cyanobacterium, Anacystis nidulans, was examined in the presence of gabaculine (5-amino-1,3-cyclohexadienyl-carboxylic acid). At 20 micromolar, this inhibitor blocked the biosynthesis of both chlorophyll and phycocyanin. Analogs of gabaculine were not effective as inhibitors of chlorophyll or phycocyanin biosynthesis. Iron- and phosphate-deficient cultures were 2- to 4-fold more sensitive to the inhibitor than were normal or nitrate-deficient cultures. Inhibition resulted in the excretion of a mixture of organic acids by the cells. δ-Aminolevulinic acid was a principle component of the mixture, identified by thin layer chromatography. Excretion of δ-aminolevulinic acid occurred following a brief lag after gabaculine addition. It remained linear for nearly 24 hours and was dependent upon illumination. However, high light inhibited excretion. Apparently, gabaculine blocks chlorophyll biosynthesis after the formation of δ-aminolevulinic acid in cyanobacteria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Batterton J. C., Van Baalen C. Phosphorus deficiency and phosphate uptake in the blue-green alga Anacystis nidulans. Can J Microbiol. 1968 Apr;14(4):341–348. doi: 10.1139/m68-056. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : II. OBSERVATIONS ON THE BIOSYNTHETIC PATHWAY IN ISOLATED ETIOCHLOROPLASTS. Plant Physiol. 1982 Jan;69(1):112–116. doi: 10.1104/pp.69.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csatorday K., Gombos Z., Szalontai B. Mn and Co toxicity in chlorophyll biosynthesis. Proc Natl Acad Sci U S A. 1984 Jan;81(2):476–478. doi: 10.1073/pnas.81.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J., Gassman M. Induction of porphyrin synthesis in etiolated bean leaves by chelators of iron. Plant Physiol. 1974 Feb;53(2):206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner G., Gorton H. L. Inhibition of phytochrome synthesis by gabaculine. Plant Physiol. 1985 Mar;77(3):540–543. doi: 10.1104/pp.77.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Metronidazole and the isolation of temperature-sensitive photosynthetic mutants in cyanobacteria. J Bioenerg Biomembr. 1980 Aug;12(3-4):277–295. doi: 10.1007/BF00744689. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Organization and Function of Chlorophyll in Membranes of Cyanobacteria during Iron Starvation. Plant Physiol. 1983 Oct;73(2):250–256. doi: 10.1104/pp.73.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. M., Pearson S. A., Smith A. J., Rogers L. J. Inhibition of chlorophyll synthesis in Hordeum vulgare by 3-amino 2,3-dihydrobenzoic acid (gabaculin). Biosci Rep. 1985 Sep;5(9):775–781. doi: 10.1007/BF01119876. [DOI] [PubMed] [Google Scholar]
- Kipe-Nolt J. A., Stevens S. E. Biosynthesis of delta-Aminolevulinic Acid from Glutamate in Agmenellum quadruplicatum. Plant Physiol. 1980 Jan;65(1):126–128. doi: 10.1104/pp.65.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipe-Nolt J. A., Stevens S. E., Jr, Stevens C. L. Biosynthesis of delta-aminolevulinic acid by blue-green algae (cyanobacteria). J Bacteriol. 1978 Jul;135(1):286–288. doi: 10.1128/jb.135.1.286-288.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Pakrasi H. B., Goldenberg A., Sherman L. A. Membrane Development in the Cyanobacterium, Anacystis nidulans, during Recovery from Iron Starvation. Plant Physiol. 1985 Sep;79(1):290–295. doi: 10.1104/pp.79.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R. J., Klein O. The determination of 4,5-dioxovaleric acid in plant preparations and a procedure for the assay of L-alanine:4,5-dioxovaleric acid aminotransferase (EC 2.6.1.43) activity. Anal Biochem. 1981 Sep 15;116(2):511–518. doi: 10.1016/0003-2697(81)90395-x. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Mechanism of the irreversible inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase by the neutrotoxin gabaculine. Biochemistry. 1977 Oct 18;16(21):4604–4610. doi: 10.1021/bi00640a012. [DOI] [PubMed] [Google Scholar]
- Spiller S. C., Castelfranco A. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : I. IN VIVO OBSERVATIONS ON IRON AND OXYGEN-DEFICIENT PLANTS. Plant Physiol. 1982 Jan;69(1):107–111. doi: 10.1104/pp.69.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]


