Abstract
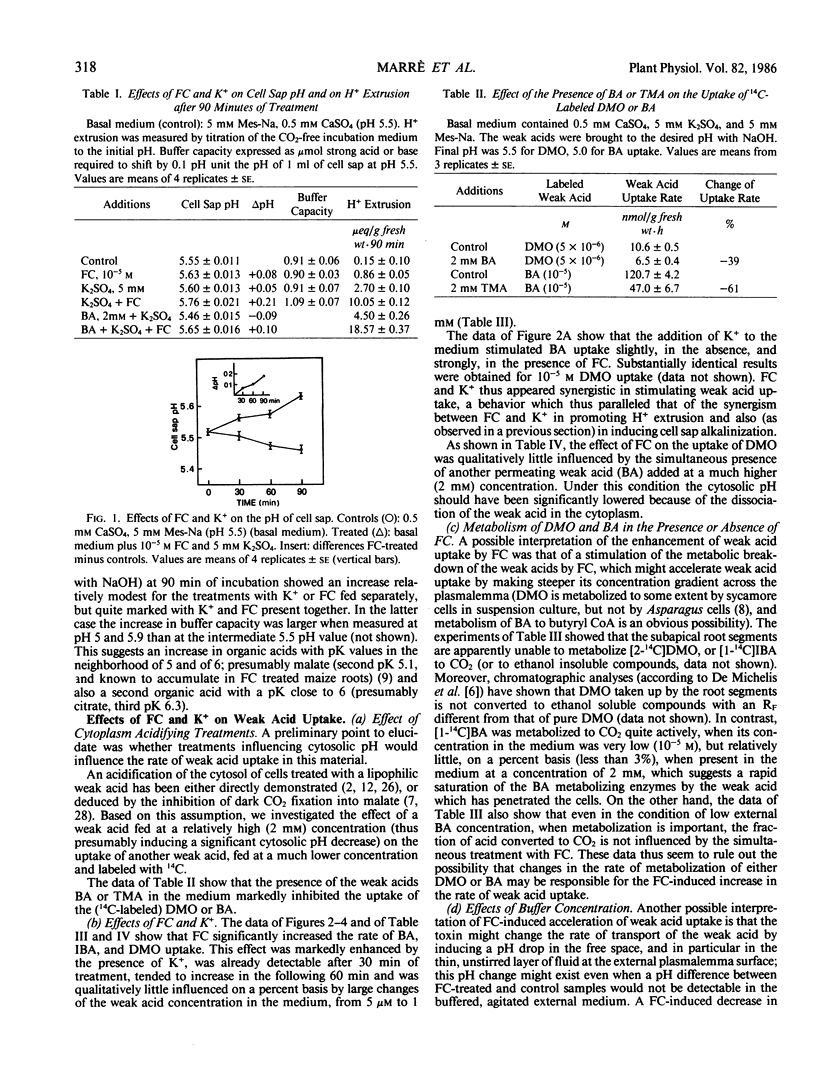
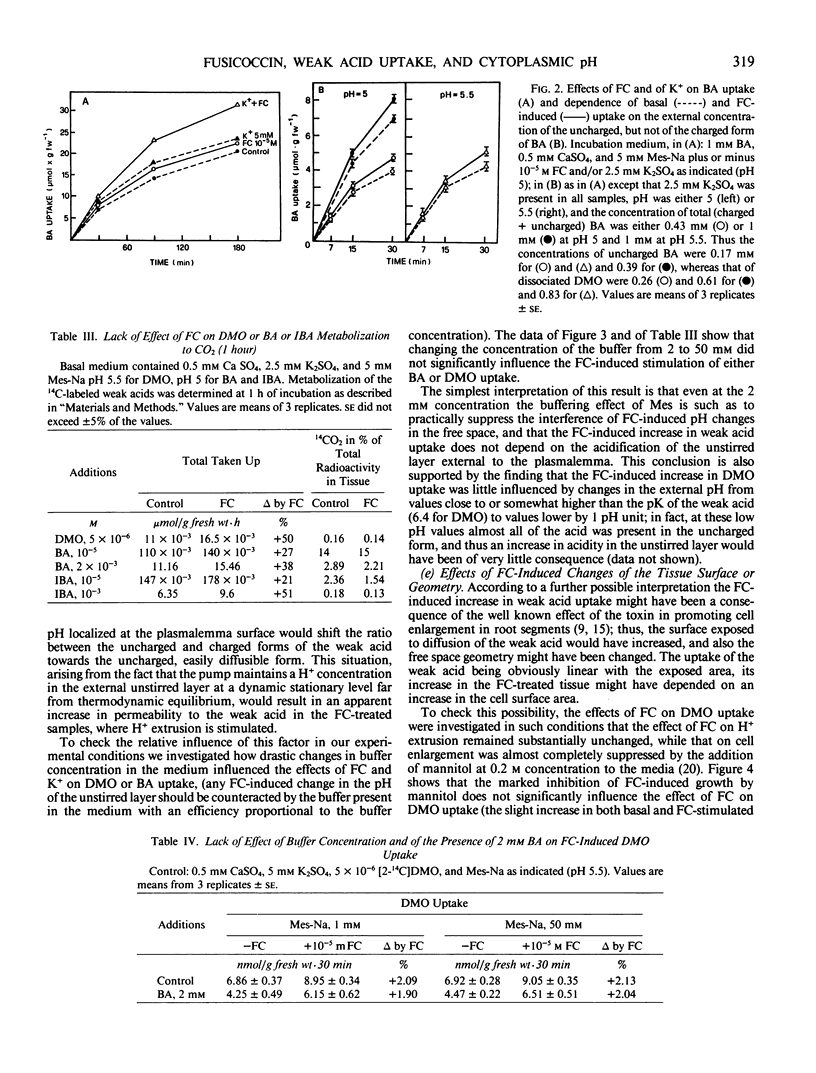
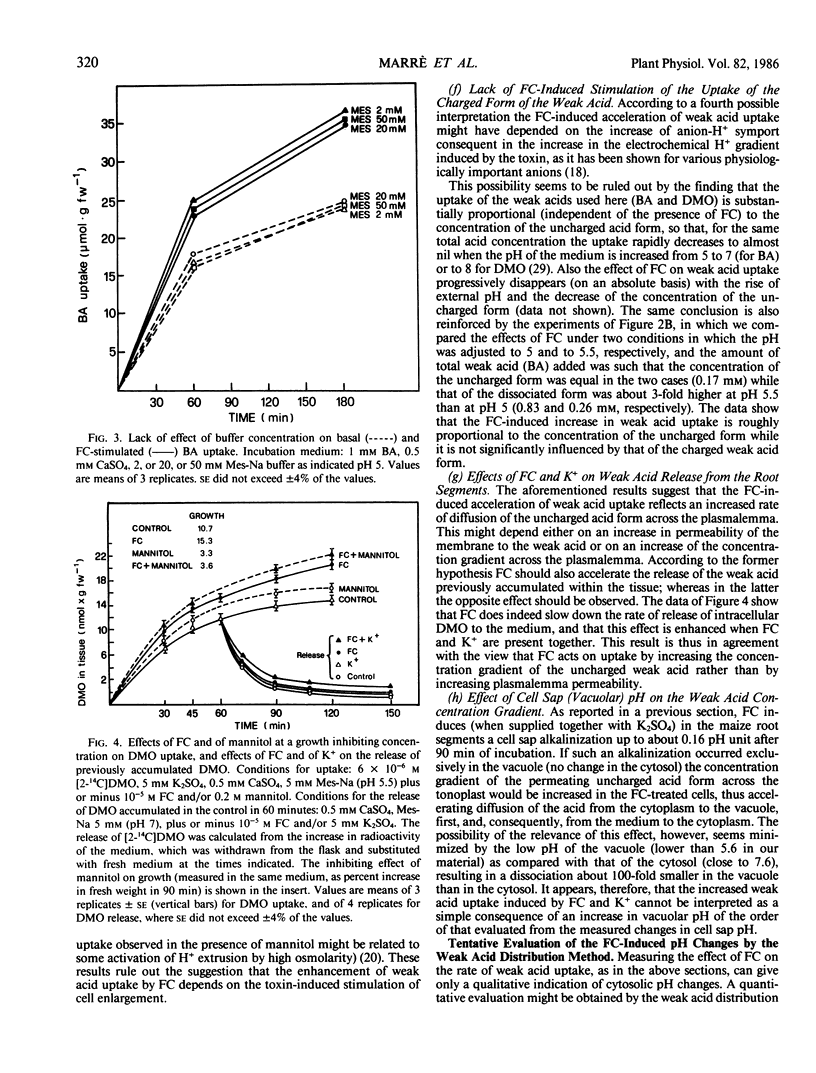
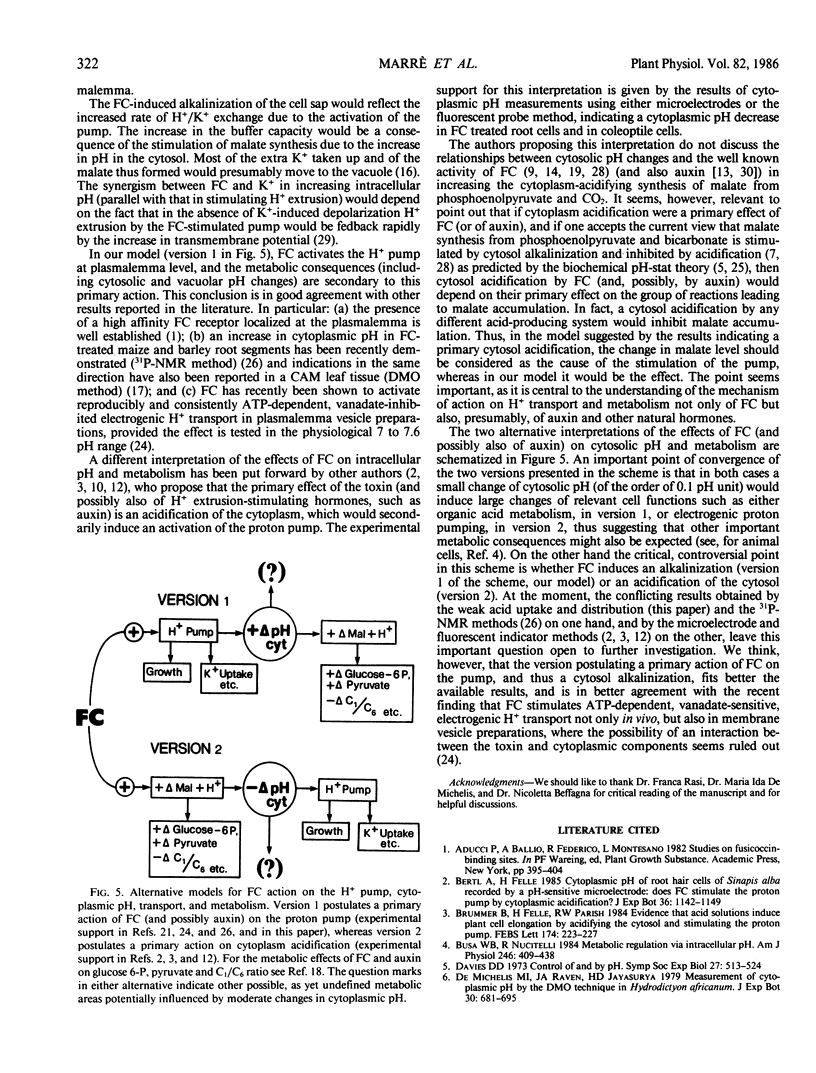
In maize root segments fusicoccin induced a consistent increase in cell sap pH (taken as representative of vacuolar pH). This effect was markedly enhanced by the presence of K+ in the medium, whereas in the absence of fusicoccin K+ did not significantly influence cell sap pH. Treatment with a weak acid at 2 mm concentration inhibited the uptake of a different (14C-labeled) weak acid fed at a lower concentration, thus suggesting that acidification of the cytoplasm inhibits weak acid uptake. Fusicoccin and K+ increased the rate of uptake of 5,5-dimethyloxazolidine-2,4-dione, butyric acid, or isobutyric acid slightly when fed separately, strongly when fed in combination. The synergism between fusicoccin and K+ in stimulating weak acid uptake was parallel to that observed for the stimulation of H+ extrusion. Application of the weak acid distribution method to a condition of `quasi-equilibrium' indicated that fusicoccin induces a cytosolic pH increase of about 0.14 unit. These results are interpreted as providing circumstantial evidence that fusicoccin- and K+- induced stimulation of H+ extrusion led to an alkalinization of the cytosol, and that other early metabolic responses, such as an increase in malate level, are a consequence of the increase in cytosolic pH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies D. D. Control of and by pH. Symp Soc Exp Biol. 1973;27:513–529. [PubMed] [Google Scholar]
- Haschke H. P., Lüttge U. Stoichiometric Correlation of Malate Accumulation with Auxin-dependent K-H Exchange and Growth in Avena Coleoptile Segments. Plant Physiol. 1975 Nov;56(5):696–698. doi: 10.1104/pp.56.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrè E., Ballarin-Denti A. The proton pumps of the plasmalemma and the tonoplast of higher plants. J Bioenerg Biomembr. 1985 Feb;17(1):1–21. doi: 10.1007/BF00744985. [DOI] [PubMed] [Google Scholar]
- Rasi-Caldogno F., Pugliarello M. C. Fusicoccin stimulates the H+-ATPase of plasmalemma in isolated membrane vesicles from radish. Biochem Biophys Res Commun. 1985 Nov 27;133(1):280–285. doi: 10.1016/0006-291x(85)91872-8. [DOI] [PubMed] [Google Scholar]
- Romani G., Marrè M. T., Bellando M., Alloatti G., Marrè E. H extrusion and potassium uptake associated with potential hyperpolarization in maize and wheat root segments treated with permeant weak acids. Plant Physiol. 1985 Nov;79(3):734–739. doi: 10.1104/pp.79.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]


