Abstract
Ketogenic diets (KDs) are actively being evaluated for their potential anticancer effects. Although KDs are generally considered safe, their safety profile when combined with chemotherapy remains unknown. It is known that a KD enhances the anticancer effect of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) in LSL-KrasLSL-G12D/+Trp53R172H/+Pdx-1-Cre (KPC) tumor-bearing mice. In the present study, whether a KD in combination with gemcitabine affected the liver safety profile in KPC mice was evaluated. For this purpose, male and female pancreatic tumor-bearing KPC mice were allocated to a control diet (CD; % kcal: 20% fat, 65% carbohydrate, 15% protein) + gemcitabine [control plus gemcitabine group (CG)] or a KD (% kcal: 84% fat, 15% protein, 1% carbohydrate) + gemcitabine [ketogenic plus gemcitabine group (KG)] for two months. After two months of treatment, no significant differences in body weight were observed between CGs and KGs. Moreover, the KD did not significantly alter the serum protein expression levels of liver enzymes, including aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase. In addition, the KD did not alter markers of liver-lipid accumulation as well as serum cholesterol and triglyceride levels, compared with the CG-treated group. Upon histologic examination, steatosis was rare, with no notable differences between treatment groups. When examining liver fatty acid composition, KD treatment significantly increased the content of saturated fatty acids and significantly decreased levels of cis-monounsaturated fatty acids compared with the CG. Finally, the KD did not affect liver markers of inflammation and oxidative stress, nor the protein expression levels of enzymes involved in ketone bodies, such as 3-hydroxy-3-methylglutaryl-CoA lyase and hidroximetilglutaril-CoA sintasa, and glucose metabolism, such as hexokinase 2, pyruvate dehydrogenase and phosphofructokinase. In summary, a KD in combination with gemcitabine appears to be safe, with no apparent hepatotoxicity and these data support the further evaluation of a KD as an adjuvant dietary treatment for pancreatic cancer.
Keywords: ketogenic diet, gemcitabine, pancreatic cancer, KPC mice, liver steatosis, liver toxicity
Introduction
Ketogenic diets (KDs) are low carbohydrate, adequate protein, high fat diets that mimic fasting and induce ketosis (1). KDs are well-established, effective and clinically approved dietary treatments for children with drug-resistant epilepsy and are used therapeutically in other neurological conditions such as Alzheimer's disease, traumatic brain injury and stroke (2,3). Moreover, they have been shown to be beneficial as adjuvant therapy in certain types of cancer, including lung, stomach and ovarian cancer (4,5). Although, preclinical evidence indicates that KDs are safe and feasible for use in cancer treatment (6), the safety of KDs in combination with chemotherapy is still unclear, so their potential use as adjuvant cancer treatment remains controversial.
The liver is a major organ for the biotransformation of xenobiotics and for the conversion of fatty acids into ketone bodies, such as β-hydroxybutyrate, acetoacetate and acetone (7). Therefore, hepatic metabolism serves a major role in substrate availability and metabolic alterations during fasting or with fasting mimicking diets such as KDs, especially when given simultaneously with chemotherapy (8–10). It was recently reported that a KD enhances the anticancer effect of gemcitabine (2′,2′-difluoro-2′-deoxycytidine), improves survival and mitigates cachexia in LSL-KrasLSL-G12D/+Trp53R172H/+Pdx-1- Cre (KPC) tumor-bearing mice (11,12). Although additional evidence on the efficacy of KDs in combination with chemotherapy exists (4,13), little is known regarding the safety of KDs with or without concomitant treatment with gemcitabine, an antineoplastic drug commonly used in pancreatic cancer treatment. While gemcitabine is generally considered safe, it has been associated with elevations in indicators of liver disease, such as serum aminotransferases [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] and with liver injury in patients with preexisting liver disease (14). Furthermore, a small number of case reports have indicated that this cytotoxic drug can cause steatohepatitis and hepatotoxicity (15–17).
Given the potential of KDs as a therapeutic diet for pancreatic cancer when combined with chemotherapy (12), comprehensive safety studies are critical in order to advance this promising treatment strategy into the clinic. Therefore, the present study performed a secondary analysis using previously collected liver and serum samples from our recent study (12), to evaluate the liver safety profile of a KD in combination with gemcitabine in KPC mice bearing pancreatic tumors.
Materials and methods
Animal studies
All animal experiments were performed according to ethical guidelines and were approved by The Animal Care and Use Committee of the University of California (approval no. 20555). KPC mice, bred at The UC Davis Animal Facility in Meyer Hall, were generated from three mouse parental strains (LSL-KrasG12D/+, LSL-Trp53R172H/+ and Pdx-1-Cre) backcrossed on the C57BL/6 strain background, as previously described (18). The genetic background of each pup was confirmed by toe DNA extraction and PCR. Briefly, genomic DNA was extracted using the Extract-N-Amp™ Tissue PCR kit (cat. no. XNAT2R; MilliporeSigma) following the manufacturer's instructions. PCR was performed for Kras, p53 and Pdx-1-Cre using the following conditions: Initial denaturation at 95°C for 2 min, followed by 37 cycles at 95°C for 0.5 min, 58°C for 1 min and 68°C for 1 min. The oligonucleotide primer sequences used were as follows: Kras 5′-CCTTTACAAGCGCACGCAGAG-3′ sense, and 5′-AGCTAGCCACCATGGCTTGAGTAAGTCTGCA-3′ anti-sense; Control p53 5′-CTTGGAGACATAGCCACACTG-3′, Mutated p53 5′-AGCTAGCCACCATGGCTTGAGTAAGTCTGCA-3′, WT p53; 5′-TTACACATCCAGCCTCTGTGG-3′, and Cre 5′-CTGGACTACATCTTGAGTTGC-3′ sense and 5′-GGTGTACGGTCAGTAAATTTG-3′ antisense. PCR products were separated on a 2% agarose gel, stained with GelRed® (cat. no. 41003; Biotium, Inc.) and visualized in a Chemidoc™ Imaging-System (Bio-Rad Laboratories, Inc.). After weaning and confirmation of the genotype by PCR, KPC mice [3 months old; weighing between 20 and 25 g (females), and 23 and 28 g (males)] were individually housed in polycarbonate cages in a room with controlled humidity (40–60%) and temperature (22–24°C), maintained on a 12 h light-dark cycle and fed LabDiet 5001 chow diet (LabDiet®) ad libitum until they were enrolled in the studies.
Dietary and chemotherapy interventions
The experimental design of the present secondary analysis has been previously described (12). Briefly, following tumor size determination using a high-resolution ultrasound imaging of the pancreas using a Vevo 2100 System (Visual Sonics; FUJIFILM Wako Pure Chemical Corporation), male and female KPC mice (4–5 mice/sex/group), with a confirmed tumor volume of ~250 mm3, were randomized to either a control group (CG; % kcal: 20% fat, 65% carb, 15% protein + gemcitabine) or a ketogenic group (KG; % kcal: 84% fat, 15% protein, 1% carb + gemcitabine). Gemcitabine was administered to the CG and KG groups at 100 mg/kg by intraperitoneal injection twice per week for 3.5 weeks (seven total injections). Lard was the main fat source in the KD, whereas in the control diet (CD) it was soybean oil (Table SI). The exact fatty acid composition of the diets is presented in Table I. The mineral mix, TD94046 was used for the CD and TD79055 was used for the KD due to the lower carbohydrate content (both from Envigo Rms, Inc.; Bioanalytical Systems, Inc.). For both diets, the CA40060 vitamin mix was used (Inotiv; Table SI). Gemcitabine (>99% 2′-deoxy-2′,2′-difluorocytidine; Gemzar; cat. no. LY-188011; Thermo Fisher Scientific, Inc.) was administered at 100 mg/kg by i.p, injection 2×/week for 3.5 weeks (for a total of 7 injections). Throughout the study, KPC mice were weighed twice a week and observed daily for signs of inactivity and presence of abdominal ascites. Endpoint criteria included the development of abdominal ascites, weight loss >20% of the initial weight, extreme weakness and inactivity. After 2 months of dietary treatment, mice were euthanized by carbon dioxide inhalation at a displacement rate of 30% vol/min and the liver was dissected, weighed and split into three parts that were either stored in liquid nitrogen, RNAlater® or 10% buffered formalin.
Table I.
Fatty acid composition in the CD and the KD.
| Fatty acids | CD, % of total fatty acids | KD, % of total fatty acids |
|---|---|---|
| SFA | 17.3 | 35.3 |
| cis-MUFA | 20.3 | 39.1 |
| n6-PUFA | 53.6 | 23.0 |
| n3-PUFA | 8.2 | 2.0 |
| n6/n3 ratio | 6.5 | 11.2 |
| Total PUFAs | 61.8 | 25.0 |
The content of SFAs, MUFAs, n3- and n6-PUFAs expressed as % of total fatty acids in the experimental diets. CD, control diet; KD, ketogenic diet; SFAs, saturated fatty acids; MUFAs, cis-monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids.
Metabolic measurements
Blood was collected via cardiac puncture after euthanasia and serum was isolated following centrifugation at 3,000 × g for 10 min at room temperature. Total serum cholesterol (cat. no. 03039773), triglycerides (cat. no. 20767107 322), ALT (cat. no. 20764957 322), AST (cat. no. 20764949 322), alkaline phosphatase (ALP; cat. no. 03333752 190), total bilirubin (cat. no. 05795397 190), albumin (cat. no. 04469658 190), creatinine (cat. no. 03263991 190), total protein (cat. no. 03183734 190) and blood urea nitrogen (cat. no. 04460715 190) were measured using COBAS INTEGRA kits (Roche Diagnostics) according to the manufacturer's instructions.
Histology
After necropsy, liver tissue was fixed in 10% (w/v) buffered formalin overnight at 4°C. Tissues were processed, embedded in paraffin and sectioned (5 µm) by routine methods. Tissue sections were stained with hematoxylin and eosin or Masson's trichrome (Chromaview; Thermo Fisher Scientific, Inc.) following standard protocols (19,20), and analyzed, blind, by a pathologist. Sections were examined using an Olympus BX46 microscope (Olympus Corporation), with ×20 and ×40 objective lenses. The liver sections were scored for the presence of macrovesicular and microvesicular steatosis and hepatocyte hypertrophy according to the method reported by Liang et al (21). Briefly, the severity of macrovesicular steatosis and microvesicular steatosis was graded based on the percentage of the total area affected, as follows: 0 (<5%), 1 (5–33%), 2 (34–66%) and 3 (>66%). Macrovesicular steatosis was defined as the displacement of the nucleus to the side by vacuoles and microvesicular steatosis was defined as the absence of this displacement. Similarly, the level of hepatocellular hypertrophy, defined as cellular enlargement >1.5× the normal hepatocyte diameter, was scored, based on the percentage of the total area affected, into the following categories: 0 (<5%), 1 (5–33%), 2 (34–66%) and 3 (>66%). The evaluation of hepatocellular hypertrophy was based on abnormal enlargement of the liver cells, irrespective of rounding of the hepatocytes and/or changes in cytoplasm or the number of vacuoles. Inflammation was scored based on the number of inflammatory cell clusters (consisting of ≥5 lymphocytes) averaged over five fields at ×200 magnification, as follows: 0 (<0.5 foci), 1 (0.5-1.0 foci), 2 (1.0-2.0 foci) and 3 (>2.0 foci) (14).
Western blot analysis
Liver tissue homogenates were prepared using RIPA buffer (Thermo Fisher Scientific, Inc.), as previously described (22). The protein concentration was determined using the Bradford method. Aliquots of total homogenates containing 25–40 µg protein were separated by SDS 8–12.5% (w/v) polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose membranes. After blocking membranes in 5% (w/v) nonfat milk for 1 h at room temperature, they were incubated overnight at 4°C with phospho-extracellular signal-regulated protein kinases 1 and 2 (Thr202/Tyr204; p-ERK1/2; cat. no. 4376; RRID: AB_331772), extracellular signal-regulated protein kinases 1 and 2 (ERK1/2; cat. no. 9102; RRID: AB_330744), phospho-protein kinase B (Ser473; p-Akt; cat. no. 4060; RRID: AB_2315049), protein kinase B (AKT; cat. no. 9272; RRID: AB_329827), AMP-activated protein kinase (AMPK; cat. no. 2795; RRID: AB_560856), phospho-AMP-activated protein kinase (Thr172; p-AMPK; cat. no. 2535; RRID: AB_331250), p-IκBα (Ser32; cat. no. 2859; RRID: AB_561111), IκBα (cat. no. 4814; RRID: AB_390781), phospo-p65 (Ser536; p-p65; cat. no. 3033; RRID: AB_331284), p65 (cat. no. 8242; RRID: AB_10859369), acetylated lysine (cat. no. 9441; RRID: AB_331805), hexokinase 2 (HK2; cat. no. 2867; RRID: AB_2232946), pyruvate dehydrogenase (PDH; cat. no. 3205; RRID: AB_2162926), phosphofructokinase (PFK; cat. no. 13123; RRID: AB_2617178), acetyl-CoA carboxylase (ACC; cat. no. 3676; RRID: AB_2219397) and toll-like receptor 2 (TLR2; cat. no. 12276; RRID: AB_2797867) primary antibodies from Cell Signaling Technology, Inc. In addition, 3-hydroxymethyl-3-methylglutaryl-CoA synthase (HMGCS; cat. no. sc-373681; RRID: AB_10947237), sterol regulatory element binding protein 1 (SREBP1; cat. no. sc-13551; RRID: AB_628282), peroxisome proliferator-activated receptor α (PPARα; cat. no. sc-398394; RRID: AB_2885073), fatty acid synthase (FAS; cat. no. sc-74540; RRID: AB_1121387), phospho-acetyl-CoA carboxylase (p-ACC; cat. no. sc-271965; RRID: AB_10710517), fibronectin (cat. no. sc-271098, RRID: AB_10608215), collagen type 1α1 chain (Col1A1; cat. no. sc-59772; RRID: AB_1121787) and toll-like receptor 4 (TLR4; cat. no. sc-293072; RRID: AB_10611320) primary antibodies were purchased from Santa Cruz Biotechnology, Inc. Finally, 3-hydroxymethyl-3-methylglutaryl-CoA lyase (HMGCL) (cat. no. 16898-1-AP; RRID: AB_2295304) primary antibodies were purchased from Proteintech Group, Inc. and 4-hydroxynonenal (4-HNE; cat. no. ab46545; RRID: AB_722490) primary antibodies were purchased from Abcam. All antibodies were prepared using a 1:1,000 dilution. The next day, membranes were washed three times with TBS-tween 0.05% (v/v) and incubated with secondary antibodies [either HRP (cat. no. 7074) or biotinylated antibodies (cat. no. 14708) from Cell Signaling Technology, Inc.; dilution, 1:2,500] for 1 h at room temperature. Finally, following another set of washes, the conjugates were incubated with the ProSignal® Pico ECL reagent (cat. no. 20–300; Genesee Scientific Corporation), visualized and quantified by chemiluminescence detection in a Chemidoc™ Imaging-System (Bio-Rad Laboratories, Inc.). β-actin (cat. no. A1978) from MilliporeSigma (Merck KGaA) or vinculin (cat. no. 13901; RRID: AB_2728768) from Cell Signaling Technology, Inc. were used as loading controls. The densitometric analysis was performed using ImageJ version 2.3.0/1.53f (National Institutes of Health).
Liver fatty acid analysis
The content of fatty acid in KPC livers of CG and KG-treated mice was measured using gas chromatography (GC). Liver samples were freeze-dried and direct-methylated with sodium methoxide as previously described (23). Prior to the methylation step, cis-10-17:1 methyl ester (Nu-Check Prep, Inc.) was added to the samples as an internal standard. Fatty acid methyl esters (FAMEs) were analyzed using a CP-Sil88 column (100 m; 25 µm ID; 0.2 µm film thickness) in a TRACE 1310 gas chromatograph (Thermo Fisher Scientific, Inc.), which was equipped with a flame-ionization detector (GC-FID; Thermo Fisher Scientific, Inc.). Each sample was analyzed twice using a 175°C plateau temperature program (23). FAMEs were quantified using chromatographic peak area and internal standard-based calculations (24).
Statistical analysis
Data are presented as the mean ± SEM; (n=4-5/sex/group). Each experiment was conducted once. Statistical analysis was performed by unpaired t-test or two-factor analysis of variance followed by Tukey's test for multiple comparisons using GraphPad Prism software (Dotmatics; version 9.2). P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of a KD in combination with gemcitabine on liver function tests in KPC mice
It has been recently reported that a KD plus gemcitabine enhances overall survival and mitigates cachexia in KPC mice compared with mice fed a CD (11,12). Based on these promising results, the present secondary analysis was performed to evaluate the impact of a KD in combination with gemcitabine on the liver safety profile in KPC mice.
Initially, the safety profile of KG in female and male KPC mice treated for 2 months was evaluated. CD + gemcitabine was selected as the CG to specifically assess the effect of a KD. No significant differences in body weight were observed between CG and KG throughout the treatment (Fig. 1A and B). Moreover, no differences in liver weight were observed between groups at the endpoint (Fig. 1C). No liver metastatic events were observed in CG- or KG-treated KPC mice at euthanasia.
Figure 1.
Effect of a KD in combination with gemcitabine on body weight, liver weight, liver enzymes, kidney markers and serum lipids in KPC mice. (A) Body weight at each gemcitabine injection, (B) final body weight and (C) liver weight at euthanasia, (D) serum levels of liver enzymes aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase and bilirubin levels. (E) Serum total protein and albumin levels. (F) Serum creatinine and blood urea nitrogen levels. (G) Serum cholesterol and triglycerides levels. Values are presented as mean ± SEM, *P<0.05. n=4-5 animals/group/sex. KPC, LSL-KrasLSL-G12D/+ Trp53R172H/+Pdx-1-Cre; CG, control plus gemcitabine group; KG, ketogenic plus gemcitabine group.
To determine whether KG affected normal liver function, the levels of serum liver enzymes AST, ALT and ALP, as well as bilirubin levels in female and male KPC mice at euthanasia were determined (Fig. 1D). After 2 months of treatment, there was no significant difference in liver enzyme and bilirubin levels between the CG- and KG-treated groups. Of note, all the mice had values within normal ranges, except one male mouse in the CG group and one male mouse in the KG group, which showed liver enzyme levels higher than the physiological range for C57BL/6 mice (physiological range, 46–221 U/l for AST, 22–133 U/l for ALT, 16–200 U/l for ALP and 0.4±0.5 mg/dl for bilirubin) (25). Moreover, no differences in serum total protein and albumin levels were observed (Fig. 1E).
Kidney function indicators were also assessed, with no significant differences in creatinine levels between the CG and KG. In contrast, higher blood urea nitrogen levels were observed in the serum of CG-treated KPC female mice, as well as when both sexes were analyzed together, compared with the KG-treated group (Fig. 1F). The serum lipid profile was measured and no significant differences in total cholesterol or triglycerides were observed between CG and KG groups (Fig. 1G).
Effect of a KD in combination with gemcitabine on liver lipid accumulation
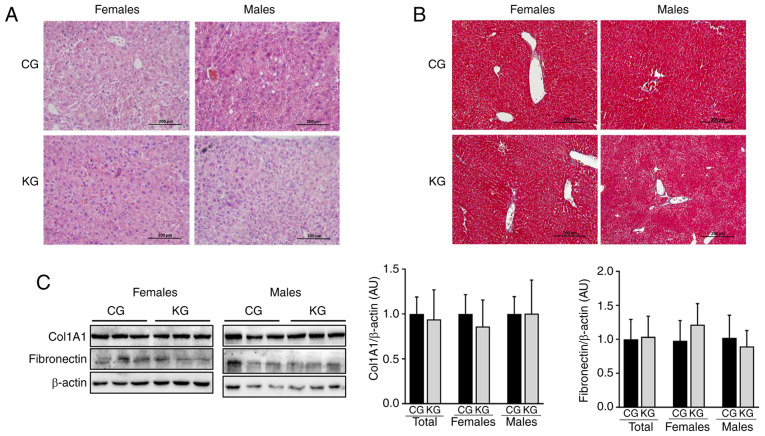
Whether a KD in combination with gemcitabine could impact hepatic lipid accumulation/steatosis was subsequently evaluated. For this purpose, histologic sections of liver from CG and KG-treated mice were evaluated (Fig. 2A and B) and the presence of macrovesicular steatosis, microvesicular steatosis and hepatocyte hypertrophy were assessed using a previously reported scoring system (21). Overall, no notable differences in macrovesicular steatosis, microvesicular steatosis, hepatocyte hypertrophy or inflammation were observed between CG- and KG-treated mice. Additionally, no histologic evidence of fibrosis was observed in either group (Fig. 2B). This was confirmed by evaluating the protein expression levels of fibronectin and Col1A1 using immunoblotting. No significant differences in fibronectin and Col1A1 levels were demonstrated in liver homogenates obtained from CG- and KG-treated mice, together or when separated by sex (Fig. 2C).
Figure 2.
A KD in combination with gemcitabine has no effect on liver lipid accumulation. (A) Hematoxylin and eosin and (B) Mason trichome staining images for liver isolated from female and male KPC mice treated with a CD or a KD. All images were digitally scanned at ×20 original magnification. (C) Immunoblotting for fibronectin and Col1A1 from liver homogenates from CG- and KG-treated female and male KPC mice following 2 months of treatment, with β-actin as the loading control. Representative images are shown. Each band represents an independent liver homogenate sample obtained from either female or male KPC mice treated with the CD or the KD. Bands were quantified and results are expressed as a proportion of the control. Values are presented as mean ± SEM. n=4-5 animals/group/sex. KPC, LSL-KrasLSL-G12D/+Trp53R172H/+Pdx-1-Cre; Col1A1, collagen type 1α1 chain; CG, control plus gemcitabine group; ketogenic plus gemcitabine group; CD, control diet; KD, ketogenic diet.
Effect of a KD in combination with gemcitabine on liver fatty acid composition
Next, whether feeding a KD plus gemcitabine could affect fatty acid profiles in the liver was evaluated. Compared with CG-treated mice, KG significantly increased the proportion of total saturated fatty acids (SFAs), mainly driven by the increased levels of stearic acid (18:0) and margaric acid (17:0) and significantly reduced the proportion of cis-monounsaturated fatty acids (c-MUFAs), including reductions in palmitoleic acid (cis9-16:1), oleic acid (cis9-18:1) and ascleptic acid (cis11-18:1). In contrast, no significant differences were demonstrated in the concentrations of n6-polyunsaturated fatty acids (PUFAs), n3-PUFAs or total PUFAs in the liver tissue of the KG mice compared with the CG mice (Fig. 3; Table SII).
Figure 3.
Effect of a ketogenic diet in combination with gemcitabine on liver FA composition. (A) SFAs, MUFAs, n-6 and n-3 PUFAs, as well as the n-6/n-3 fatty acid ratio, concentrations (% of total FAs) of select FAs, (B) palmitoleic acid (cis9-16:1) and oleic acid (cis9-18:1), (C) ascleptic acid (cis11-18:1), gondoleic acid (cis9-20:1), margarolic acid (cis9-17:1), eicosanoic acid (cis11-20:1), dihomo-g-linolenic acid (20:3, n-6), α-linolenic acid (18:3n-3), eicosapentaenoic acid (20:5n-3) and docosapentaenoic acid (22:5n-3) and (D) stearic acid (18:0), margaric acid (17:0), arachidonic acid (20:4n-6), adrenic acid (22:4n-6) and docosahexaenoic acid (22:6n-3) in liver homogenates isolated from CG- and KG-treated KPC female and male mice following 2 months of treatment. Values are presented as mean ± SEM, *P<0.05 and **P<0.01 n=5 animals/group/sex. SFAs, short chain fatty acids; FAs, fatty acids; MUFAs, monounsaturated fatty acids; PUFAs, polyunsaturated fatty acids; CG, control plus gemcitabine group; KG, ketogenic plus gemcitabine group; KPC, LSL-KrasLSL-G12D/+Trp53R172H/+ Pdx-1-Cre.
When analyzing individual fatty acids, it was observed that dihomo-g-linolenic acid (20:3, n-6) from the n6 family, α-linolenic acid (18:3n-3), eicosapentaenoic acid (20:5n-3) and docosapentaenoic acid (22:5n-3) from the n3 family were significantly reduced in KG group in contrast to CG group. At the same time, arachidonic acid (20:4n-6), adrenic acid (22:4n-6) from the n6 family and docosahexaenoic acid (22:6n-3) from the n3 family were significantly increased in KG-treated livers compared with the CG group (Table SII).
Effect of a KD in combination with gemcitabine on de novo lipogenesis regulatory proteins
Next, whether KG could influence the expression levels of sterol regulatory element-binding proteins (SREBPs) were evaluated, which together with FAS and ACC enzymes are central regulators of de novo lipogenesis (26–28). No significant differences were observed in either SREBP1 protein expression levels or ACC activation between CG- and KG-treated mice. However, KG treatment significantly reduced FAS protein expression levels in the livers of male mice, compared with CG-treated mice (Fig. 4). Moreover, no significant differences were observed in the liver protein expression levels of PPARα, a key nuclear receptor regulating de novo lipogenesis (29), between CG- and KG-treated mice, nor by sex (4–5 mice/group).
Figure 4.

Effect of a KD in combination with gemcitabine on enzymes involved in de novo fatty acids synthesis. Immunoblots of SREBP-1, FAS, p-ACC/ACC, PPARα, pAMPK/AMPK and pAKT/AKT from liver homogenates from CG- and KG-treated female and male KPC mice following 2 months of treatment. Representative images are shown. Each band represents an independent liver homogenate sample obtained from either female or male KPC mice treated with CD or KD. Bands were quantified and values normalized to the non-phosphorylated protein (ACC, AKT and AMPK) or β-actin levels (SREBP-1, FAS and PPARα). Results are expressed as a proportion of the control, *P<0.05, values are presented as mean ± SEM with 4 animals/group/sex. CD, control diet; KD, ketogenic diet; CG, control plus gemcitabine group; KG, ketogenic plus gemcitabine group; KPC, LSL-KrasLSL-G12D/+Trp53R172H/+Pdx-1-Cre; SREBP-1, sterol regulatory element binding protein 1; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; ACC, acetyl-CoA carboxylase; PPARα, peroxisome proliferator-activated receptor α; AMPK, AMP-activated protein kinase; AMPK, AMP-activated protein kinase; AKT, protein kinase B; AKT, protein kinase B; p, phosphorylated.
Finally, given that AMPK and AKT can stimulate de novo lipid synthesis by activating SREBP (27,28), whether KG could affect AMPK and AKT phosphorylation levels was assessed. There were no significant differences in AMPK and AKT phosphorylation levels demonstrated between CG- and KG-treated mice (Fig. 4).
Effects of a KD in combination with gemcitabine on ketogenesis and glucose metabolism
Given that the liver is a major organ involved in the metabolism of ketone bodies and glucose (30), the regulation of enzymes involved in ketogenesis (HMGCL and HMGCS) and glucose metabolism (HK2, PFK and PDH) following KG treatment was investigated. No significant differences in HMGCL, HMGCS, HK2, PFK and PDH hepatic protein expression levels were demonstraed between CG- and KG-treated mice together or separated by sex (Fig. 5A and B).
Figure 5.
Effect of a KD in combination with gemcitabine on ketogenesis and glucose metabolism. Immunoblotting of (A) HMGCL and HMGCS, (B) HK2, PFK and PDH and (C) acetylated lysine from liver homogenates from CG- and KG-treated female and male KPC mice following 2 months of treatment. The loading controls were vinculin or β-actin. Representative images are shown. Each band represents an independent liver homogenate sample obtained from either female or male KPC mice treated with a CD or KD. Bands were quantified and results are presented as a proportion of the control; *P<0.05. Values are presented as mean ± SEM with 5 animals/group/sex. CD, control diet; KD, ketogenic diet; CG, control plus gemcitabine group; KG, ketogenic plus gemcitabine group; KPC, LSL-KrasLSL-G12D/+Trp53R172H/+Pdx-1-Cre; HMGCL, 3-hydroxymethyl-3-methylglutaryl-CoA lyase; HMGCS, 3-hydroxymethyl-3-methylglutaryl-CoA synthase, HK2, hexokinase 2; PFK, phosphofructokinase; PDH, pyruvate dehydrogenase.
Acetylation may serve a key role in the coordination of different metabolic pathways in response to extracellular conditions, including nutrient availability (31). Since the liver is highly exposed to lysine acetylation (32), whether a KD in combination with gemcitabine affected acetylation was investigated. After 2 months of treatment, significantly increased lysine acetylation levels in the liver of female, but not male, KG-treated mice when compared with CG-treated mice were observed (Fig. 5C).
Effect of a KD in combination with gemcitabine on markers of inflammation and oxidative stress in the liver of KPC mice
Activation of TLR signaling is key during liver inflammation processes, with TLR4 and TLR2 serving crucial roles in the progression of NASH (33). Therefore, if a KD could impact the activation of TLR2 and TLR4 was evaluated. Moreover, since TLR cascades can lead to the activation of NF-κB and mitogen-activated protein kinases (MAPKs) pathways, which serve central roles in inflammation, the phosphorylation levels of IκBα, p65 and ERK1/2 were measured. After 2 months of intervention, no significant differences in TLR4, TLR2 levels nor in the downstream IκBα and ERK1/2 phosphorylation levels were observed in the livers of KG- or CG-treated male and female mice (Fig. 6A and B). A significant increase in p65 phosphorylation was observed in all and male mice in the KG when compared with the KG; however, no significant difference was demonstrated between KG and CG within the female group (Fig. 6B).
Figure 6.
Effect of a KD in combination with gemcitabine on markers of inflammation and oxidative stress. Immunoblotting of (A) TLR2 and TLR4, (B) p-IκBα, IκBα, p-p65, p65, p-ERK and ERK and (C) 4-HNE from liver homogenates isolated from CG- and KG-treated female and male KPC mice following 2 months of treatment. The loading control used was β-actin. Representative images are shown. Each band represents an independent liver homogenate sample obtained from either female or male KPC mice treated with a CD or KD. Bands were quantified and values normalized to (A) the non-phosphorylated protein and (B and C) β-actin levels. Results are expressed as a proportion of the control. *P<0.05. Values are presented as mean ± SEM. n=4 animals/group/sex. CD, control diet; KD, ketogenic diet; CG, control plus gemcitabine group; KG, ketogenic plus gemcitabine group; KPC, LSL-KrasLSL-G12D/+ Trp53R172H/+Pdx-1-Cre; TLR, toll-like receptor; ERK, extracellular signal-regulated protein kinase; ERK, extracellular signal-regulated protein kinase; 4-HNE, 4-hydroxynonenal; p, phosphorylated.
Given that oxidative stress serves a major role in the development of liver injury (34), next 4-HNE levels, a main lipid peroxidation product that displays increased levels with oxidative stress (35), were measured. No significant differences in 4-HNE levels were observed between KG- and CG for all mice or those separated by sex (Fig. 6C).
Discussion
The characterization of the safety profile of KDs concomitant with chemotherapeutic treatments for pancreatic cancer is critical in order to develop clinical recommendations. Data on the overall safety and feasibility of KDs indicate that this diet can be tolerated by patients with cancer (36). Nevertheless, the safety of KDs when administered with standard cancer treatment modalities in patients with pancreatic cancer is still unknown. Gemcitabine is a main chemotherapeutic agent used for the treatment of pancreatic cancer (37). Although it is considered generally safe, it can cause adverse hepatic events such as elevations in serum ALT, ALP and bilirubin (38,39). A case report previously reported that gemcitabine monotherapy caused hepatic failure in a patient with advanced pancreatic cancer after pancreaticoduodenectomy (40).
Several human studies have reported the benefits of using a KD in pancreatic cancer treatment. For instance, certain randomized controlled trials have reported that a KD may improve the quality of life and overall survival of patients (41,42). Additionally, it has been suggested that a KD might help to reduce tumor growth and slow disease progression in certain cases (43–45). It was recently reported that when combined with the chemotherapeutic agent gemcitabine, a KD increases overall survival in the autochthonous-clinically relevant KPC mouse model, without any signs of intolerance to the fat content of the diet (12). Therefore, the liver safety profile of a KD in combination with gemcitabine in KPC tumor-bearing mice was evaluated. To the best of our knowledge, this is the first study to evaluate the liver safety of a KD in combination with gemcitabine.
In the present study, multiple parameters of liver toxicity were assessed in pancreatic tumor-bearing mice treated with a KD plus gemcitabine. Overall, there were no changes in liver enzymes nor in markers of kidney function with the KG regimen. Consistent with the findings of the present study, a previous study reported normal liver and kidney function tests were observed in rats after being fed a KD for 60 days (46). A recent study reported that liver function markers differ among groups fed lard, soybean oil or a blend of both, with the levels of AST and ALT being lower in subjects that consumed the blend oil for 12 weeks compared with those in subjects in the pure soybean oil or lard groups (47). The KD composition used in the present study was prepared primarily with lard (84%) and combined with soybean oil, while there was no lard in the CD, which should be taken into consideration for further research.
The effect of KDs on serum lipids has been inconsistent among previous studies. In a study where healthy male mice were given a KD for 22 weeks, cholesterol and triglycerides levels were increased and signs of hepatic steatosis were observed (48). On the other hand, a KD led to improvements in total cholesterol and triglycerides in women who were overweight (49). In the present study, a KD plus gemcitabine had no effect on serum cholesterol nor on triglyceride levels.
Long term high-fat diets can induce liver steatosis, which is a precursor of non-alcoholic fatty liver disease (NAFLD) (50). Given the high fat content of a KD, this could be a potential concern. Nevertheless, some controversy exists as to whether a KD predisposes NAFDL development. In patients with NAFLD, a KD given for 6 days improved metabolic abnormalities (51). Furthermore, a KD prevented the development of steatosis in obese mice (52). In the present study, a KD did not alter hepatic lipid accumulation, nor did it affect the protein or enzyme levels related to de novo lipogenesis, ketogenesis or glucose metabolism. Moreover, a KD plus gemcitabine treatment failed to induce macrovesicular or microvesicular steatosis, hepatocyte hypertrophy or fibrosis. Inflammatory and oxidative stress markers, two known factors that can contribute to the pathogenesis of NAFLD (53,54), were not affected either.
Hepatic fatty acid composition may affect steatosis development (55,56). In particular, low hepatic n6- and n3-PUFAs could contribute to steatosis and steatohepatitis (57). In the present study, it was observed that a KD plus gemcitabine did not affect hepatic levels of n6-PUFA, n3-PUFA nor total PUFAs (n6 + n3) compared with CG mice. On the other hand, significant differences in the composition of numerous intrahepatic fatty acids following KG-treatment, with lower MUFA levels, but higher SFAs were observed. Additional studies are warranted to elucidate the potential impact of each individual fatty acid affected by KG and their potential role in steatosis risk or prevention.
Lysine acetylation/deacetylation mediated by histone acetylases and histone deacetylases (HDACs) is a regulator of signaling pathways involved in cancer progression (58). Moreover, it serves a role in metabolic processes involved in liver disease, with certain HDAC inhibitors considered candidates for hepatocellular carcinoma treatment (59). β-hydroxybutyrate is a known HDAC inhibitor (60). In aging mice, both survival and total acetyl-lysine levels have been reported to be significantly increased in the liver of KD-fed males compared with controls (61). Hutfles et al (62), also reported an increase in cytosolic and mitochondrial acetylation in the livers of KD-fed male C57Bl/6J mice in contrast to those fed standard chow diet. To the best of our knowledge, the present study is the first to evaluate lysine acetylation in the livers of KD fed, gemcitabine treated, pancreatic tumor bearing, male and female mice. It was observed that a KD combined with gemcitabine significantly increased liver lysine acetylation in females, but not males, when compared with CG-treated mice. Interestingly, previously published data elucidated sex-dependent effects of KG treatment in the skeletal muscle of KPC animals (11), so the results of the present study highlight the importance of researching the multi-organ effects of treatments in both males and females. Unfortunately, as the research design of the present study did not include KPC mice fed with a KD alone, the exploration of the exact contribution of the KD to this effect was impeded. In addition, another limitation is that the analysis was performed using previously collected samples and the number of samples/group/sex used were not powered for this particular study. Being underpowered potentially explains why additional differences between the CGs and KGs were not demonstrated. Nevertheless, further studies are warranted to explore the specific role of KDs, without the confounding effect of gemcitabine, in the hepatic modulation of protein acetylation and the potential impact of biological sex suggested by these data.
In summary, the findings of the present study indicate that a KD in combination with gemcitabine appears to be safe in male and female mice bearing pancreatic tumors. However, it is important to stress that the liver safety profile of a specific KD with a specific dose of gemcitabine over two months was evaluated. Differences in diet composition (macronutrient distribution and/or amount and type of fats), length of the treatment and the type of adjuvant drug administered could alter the liver safety profile when incorporating a KD to the treatment strategy and require further, future study. Moreover, the present study evaluated the safety of a KD in combination with gemcitabine. Additional studies are warranted to evaluate the safety of this dietary regimen in combination with other chemotherapeutics used clinically (such as, nab-paclitaxel, 5-FU, irinotecan and oxaliplatin). Another limitation of the present study is the lack of evaluation of the liver safety of a KD alone, without the confounding effect of the chemotherapeutic agent, that would allow for the direct hepatic safety effect of this dietary intervention to be evaluated in mice bearing pancreatic tumors. Thus, future studies should focus on identifying a standardized treatment protocol that includes the composition, length and regimen for a KD (63), which would assist in translating this promising dietary treatment strategy safely into the clinic.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This research was funded by The University of California, Davis; The UCD Comprehensive Cancer center (ELEMENTS initiative); The University of California Cancer Research Coordinating Committee (award no. C23CR5560) and NIFA-USDA (grant no. CA-D-NTR-2397-H). This research was also supported by the Biorepository Shared Resources, funded by the UC Davis Comprehensive Cancer Center Support Grant awarded by the National Cancer Institute (grant no. P30CA093373).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
NEC and CRL performed conceptualization, experiments, visualization, investigation, writing of the original draft, reviewing and editing. PV analyzed the fatty acids and related data, and reviewed and edited the manuscript. KM performed histological analysis, reviewed and edited the manuscript. GGM performed conceptualization, investigation, writing, reviewing and editing of manuscript, and acquired funding. NEC and CRL confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
All animal experiments were performed according to ethical guidelines and were approved by the University of California, Davis Animal Care and Use Committee (Davis, USA; approval no. 20555).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhu H, Bi D, Zhang Y, Kong C, Du J, Wu X, Wei Q, Qin H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. 2022;7:11. doi: 10.1038/s41392-021-00831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong X, Deng Y, Liu L, Tang X, Yu T, Gan J, Cai Q, Luo R, Xiao N. Clinical implementation of ketogenic diet in children with drug-resistant epilepsy: Advantages, disadvantages, and difficulties. Seizure. 2022;99:75–81. doi: 10.1016/j.seizure.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JG, Veznedaroglu E. Ketogenic diet for malignant gliomas: A review. Curr Nutr Rep. 2020;9:258–263. doi: 10.1007/s13668-020-00332-2. [DOI] [PubMed] [Google Scholar]
- 4.Cortez NE, Mackenzie GG. Ketogenic diets in pancreatic cancer and associated cachexia: Cellular mechanisms and clinical perspectives. Nutrients. 2021;13:3202. doi: 10.3390/nu13093202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang WH, Wang WQ, Han X, Gao HL, Li TJ, Xu SS, Li S, Xu HX, Li H, Ye LY, et al. Advances on diagnostic biomarkers of pancreatic ductal adenocarcinoma: A systems biology perspective. Comput Struct Biotechnol J. 2020;18:3606–3614. doi: 10.1016/j.csbj.2020.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Zhang H, Dai Z. Cancer treatment with the ketogenic diet: A systematic review and meta-analysis of animal studies. Front Nutr. 2021;8:594408. doi: 10.3389/fnut.2021.594408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–E1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 8.Bertholdt L, Gudiksen A, Jessen H, Pilegaard H. Impact of skeletal muscle IL-6 on regulation of liver and adipose tissue metabolism during fasting. Pflugers Arch. 2018;470:1597–1613. doi: 10.1007/s00424-018-2185-1. [DOI] [PubMed] [Google Scholar]
- 9.Cunha GM, Guzman G, Correa De Mello LL, Trein B, Spina L, Bussade I, Marques Prata J, Sajoux I, Countinho W. Efficacy of a 2-month very low-calorie ketogenic diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Front Endocrinol (Lausanne) 2020;11:607. doi: 10.3389/fendo.2020.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes Rev. 2020;21:e13024. doi: 10.1111/obr.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez NE, Pathak S, Rodriguez Lanzi C, Hong BV, Crone R, Sule R, Wang F, Chen S, Gomes AV, Baar K, Mackenzie GG. A ketogenic diet in combination with gemcitabine mitigates pancreatic cancer-associated cachexia in male and female KPC mice. Int J Mol Sci. 2023;24:10753. doi: 10.3390/ijms241310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortez NE, Rodriguez Lanzi C, Hong BV, Xu J, Wang F, Chen S, Ramsey JJ, Pontifex MG, Müller M, Vauzour D, et al. A ketogenic diet in combination with gemcitabine increases survival in pancreatic cancer KPC mice. Cancer Res Commun. 2022;2:951–965. doi: 10.1158/2767-9764.CRC-22-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotti F, Terranova C, Luvero D, Bartolone M, Messina G, Feole L, Cianci S, Scaletta G, Marchetti C, Di Donato V, et al. Diet and chemotherapy: The effects of fasting and ketogenic diet on cancer treatment. Chemotherapy. 2020;65:77–84. doi: 10.1159/000510839. [DOI] [PubMed] [Google Scholar]
- 14.Hailan WAQ, Abou-Tarboush FM, Al-Anazi KM, Ahmad A, Qasem A, Farah MA. Gemcitabine induced cytotoxicity, DNA damage and hepatic injury in laboratory mice. Drug Chem Toxicol. 2020;43:158–164. doi: 10.1080/01480545.2018.1504957. [DOI] [PubMed] [Google Scholar]
- 15.Stellman A, Loke MM, Mann S. Acute liver failure secondary to gemcitabine. BMJ Case Rep. 2010;2010:bcr1220081371. doi: 10.1136/bcr.12.2008.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coeman DC, Verbeken EK, Nackaerts KL, Demedts MG, Vansteenkiste JF. A fatal case of cholestatic liver failure probably related to gemcitabine. Ann Oncol. 2000;11:1503. doi: 10.1023/A:1026514527313. [DOI] [PubMed] [Google Scholar]
- 17.Dobbie M, Hofer S, Oberholzer M, Herrmann R. Veno-occlusive disease of the liver induced by gemcitabine. Ann Oncol. 1998;9:681. doi: 10.1023/A:1008225930573. [DOI] [PubMed] [Google Scholar]
- 18.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014:655–658. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- 20.Van De Vlekkert D, Machado E, d'Azzo A. Analysis of generalized fibrosis in mouse tissue sections with masson's trichrome staining. Bio Protoc. 2020;10:e3629. doi: 10.21769/BioProtoc.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang W, Menke AL, Driessen A, Koek GH, Lindeman JH, Stoop R, Havekes LM, Kleemann R, van den Hoek AM. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS One. 2014;9:e115922. doi: 10.1371/journal.pone.0115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez Lanzi C, Perdicaro DJ, Antoniolli A, Fontana AR, Miatello RM, Bottini R, Vazquez Prieto MA. Grape pomace and grape pomace extract improve insulin signaling in high-fat-fructose fed rat-induced metabolic syndrome. Food Funct. 2016;7:1544–1553. doi: 10.1039/C5FO01065A. [DOI] [PubMed] [Google Scholar]
- 23.Dugan MER, Kramer JKG, Robertson WM, Meadus WJ, Aldai N, Rolland DC. Comparing subcutaneous adipose tissue in beef and muskox with emphasis on trans 18:1 and conjugated linoleic acids. Lipids. 2007;42:509–518. doi: 10.1007/s11745-007-3051-7. [DOI] [PubMed] [Google Scholar]
- 24.Vahmani P, Rolland DC, McAllister TA, Block HC, Proctor SD, Guan LL, Prieto N, López-Campos Ó, Aalhus JL, Dugan MER. Effects of feeding steers extruded flaxseed on its own before hay or mixed with hay on animal performance, carcass quality, and meat and hamburger fatty acid composition. Meat Sci. 2017;131:9–17. doi: 10.1016/j.meatsci.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Loeb WF, Quimby FW. Taylor and Francis; Philadelphia: 1999. The clinical chemistry of laboratory animals. [Google Scholar]
- 26.Gosmain Y, Dif N, Berbe V, Loizon E, Rieusset J, Vidal H, Lefai E. Regulation of SREBP-1 expression and transcriptional action on HKII and FAS genes during fasting and refeeding in rat tissues. J Lipid Res. 2005;46:697–705. doi: 10.1194/jlr.M400261-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Guo S. Insulin signaling, resistance, and the metabolic syndrome: Insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016;61:1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Wang T, Xia J, Yao W, Huang F. Enzymatic and nonenzymatic protein acetylations control glycolysis process in liver diseases. FASEB J. 2019;33:11640–11654. doi: 10.1096/fj.201901175R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roh YS, Seki E. Toll-like receptors in alcoholic liver disease, non-alcoholic steatohepatitis and carcinogenesis. J Gastroenterol Hepatol. 2013;28((Suppl 1)):S38–S42. doi: 10.1111/jgh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20:8082–8091. doi: 10.3748/wjg.v20.i25.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro JP, Jung T, Grune T, Siems W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic Biol Med. 2017;111:309–315. doi: 10.1016/j.freeradbiomed.2016.10.497. [DOI] [PubMed] [Google Scholar]
- 36.Tan-Shalaby J. Ketogenic diets and cancer: Emerging evidence. Fed Pract. 2017;34((Suppl 1)):37S–42S. [PMC free article] [PubMed] [Google Scholar]
- 37.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.So E, Crees ZD, Crites D, Wang-Gillam A. Digital ischemia and necrosis: A rarely described complication of gemcitabine in pancreatic adenocarcinoma. J Pancreat Cancer. 2017;3:49–52. doi: 10.1089/pancan.2017.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2012. National Institute of Diabetes and Digestive Kidney Diseases: Gemcitabine LiverTox: Clinical and research information on drug-induced liver injury. [Google Scholar]
- 40.Okada T, Egawa S, Motoi F, Yamamoto K, Ottomo S, Sakata N, Rikiyama T, Katayose Y, Unno M. Severe cholestatic liver failure associated with gemcitabine adjuvant monotherapy for pancreatic cancer. Clin J Gastroenterol. 2011;4:391–395. doi: 10.1007/s12328-011-0257-2. [DOI] [PubMed] [Google Scholar]
- 41.Klement RJ, Weigel MM, Sweeney RA. A ketogenic diet consumed during radiotherapy improves several aspects of quality of life and metabolic health in women with breast cancer. Clin Nutr. 2021;40:4267–4274. doi: 10.1016/j.clnu.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Klement RJ, Champ CE, Kämmerer U, Koebrunner PS, Krage K, Schäfer G, Weigel M, Sweeney RA. Impact of a ketogenic diet intervention during radiotherapy on body composition: III-final results of the KETOCOMP study for breast cancer patients. Breast Cancer Res. 2020;22:94. doi: 10.1186/s13058-020-01331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr Metab (Lond) 2011;8:54. doi: 10.1186/1743-7075-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, TeSlaa T, Ng S, Nofal M, Wang L, Lan T, Zeng X, Cowan A, McBride M, Lu W, et al. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med. 2022;3:119–136. doi: 10.1016/j.medj.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zahra A, Fath MA, Opat E, Mapuskar KA, Bhatia SK, Ma DC, Rodman SN, III, Snyders TP, Chenard CA, Eichenberger-Gilmore JM, et al. Consuming a ketogenic diet while receiving radiation and chemotherapy for locally advanced lung cancer and pancreatic cancer: The university of iowa experience of two phase 1 clinical trials. Radiat Res. 2017;187:743–754. doi: 10.1667/RR14668.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arsyad A, Idris I, Rasyid AA, Usman RA, Faradillah KR, Latif WOU, Lubis ZI, Aminuddin A, Yustisia I, Djabir YY. Long-term ketogenic diet induces metabolic acidosis, anemia, and oxidative stress in healthy wistar rats. J Nutr Metab. 2020;2020:3642035. doi: 10.1155/2020/3642035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Z, Yuan J, Wen P, Guo X, Li K, Wang Y, Liu R, Guo Y, Li D. Effect of lard or plus soybean oil on markers of liver function in healthy subjects: A randomized controlled-feeding trial. Foods. 2023;12:1894. doi: 10.3390/foods12091894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellenbroek JH, van Dijck L, Töns HA, Rabelink TJ, Carlotti F, Ballieux BE, de Koning EJ. Long-term ketogenic diet causes glucose intolerance and reduced β- and α-cell mass but no weight loss in mice. Am J Physiol Endocrinol Metab. 2014;306:E552–E558. doi: 10.1152/ajpendo.00453.2013. [DOI] [PubMed] [Google Scholar]
- 49.Tragni E, Vigna L, Ruscica M, Macchi C, Casula M, Santelia A, Catapano AL, Magni P. Reduction of cardio-metabolic risk and body weight through a multiphasic very-low calorie ketogenic diet program in women with overweight/obesity: A study in a real-world setting. Nutrients. 2021;13:1804. doi: 10.3390/nu13061804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Yakov G, Alao H, Haydek JP, Fryzek N, Cho MH, Hemmati M, Samala V, Shovlin M, Dunleavy K, Wilson W, et al. Development of hepatic steatosis after chemotherapy for non-hodgkin lymphoma. Hepatol Commun. 2018;3:220–226. doi: 10.1002/hep4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luukkonen PK, Dufour S, Lyu K, Zhang XM, Hakkarainen A, Lehtimäki TE, Cline GW, Petersen KF, Shulman GI, Yki-Järvinen H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2020;117:7347–7354. doi: 10.1073/pnas.1922344117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okuda T, Morita N. A very low carbohydrate ketogenic diet prevents the progression of hepatic steatosis caused by hyperglycemia in a juvenile obese mouse model. Nutr Diabetes. 2012;2:e50. doi: 10.1038/nutd.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2018;2018:9547613. doi: 10.1155/2018/9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao B, Tsukamoto H. Inflammation in alcoholic and nonalcoholic fatty liver disease: Friend or foe? Gastroenterology. 2016;150:1704–1709. doi: 10.1053/j.gastro.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berná G, Romero-Gomez M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020;40((Suppl 1)):S102–S108. doi: 10.1111/liv.14360. [DOI] [PubMed] [Google Scholar]
- 56.Musso G, Cassader M, Paschetta E, Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology. 2018;155:282–302.e8. doi: 10.1053/j.gastro.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 57.Allard JP, Aghdassi E, Mohammed S, Raman M, Avand G, Arendt BM, Jalali P, Kandasamy T, Prayitno N, Sherman M, et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): A cross-sectional study. J Hepatol. 2008;48:300–307. doi: 10.1016/j.jhep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Ding P, Ma Z, Liu D, Pan M, Li H, Feng Y, Zhang Y, Shao C, Jiang M, Lu D, et al. Lysine acetylation/deacetylation modification of immune-related molecules in cancer immunotherapy. Front Immunol. 2022;13:865975. doi: 10.3389/fimmu.2022.865975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Q, Zhang Z, Li J, Xu F, Zhang B, Liu M, Liu Y, Chen H, Yang J, Zhang J. Lysine acetylome study of human hepatocellular carcinoma tissues for biomarkers and therapeutic targets discovery. Front Genet. 2020;11:572663. doi: 10.3389/fgene.2020.572663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25:42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2017;26:539–546.e5. doi: 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hutfles LJ, Wilkins HM, Koppel SJ, Weidling IW, Selfridge JE, Tan E, Thyfault JP, Slawson C, Fenton AW, Zhu H, Swerdlow RH. A bioenergetics systems evaluation of ketogenic diet liver effects. Appl Physiol Nutr Metab. 2017;42:955–962. doi: 10.1139/apnm-2017-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA, Schoenfeld JD, Buatti JM, Spitz DR, Fath MA. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014;2:963–970. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.