Abstract
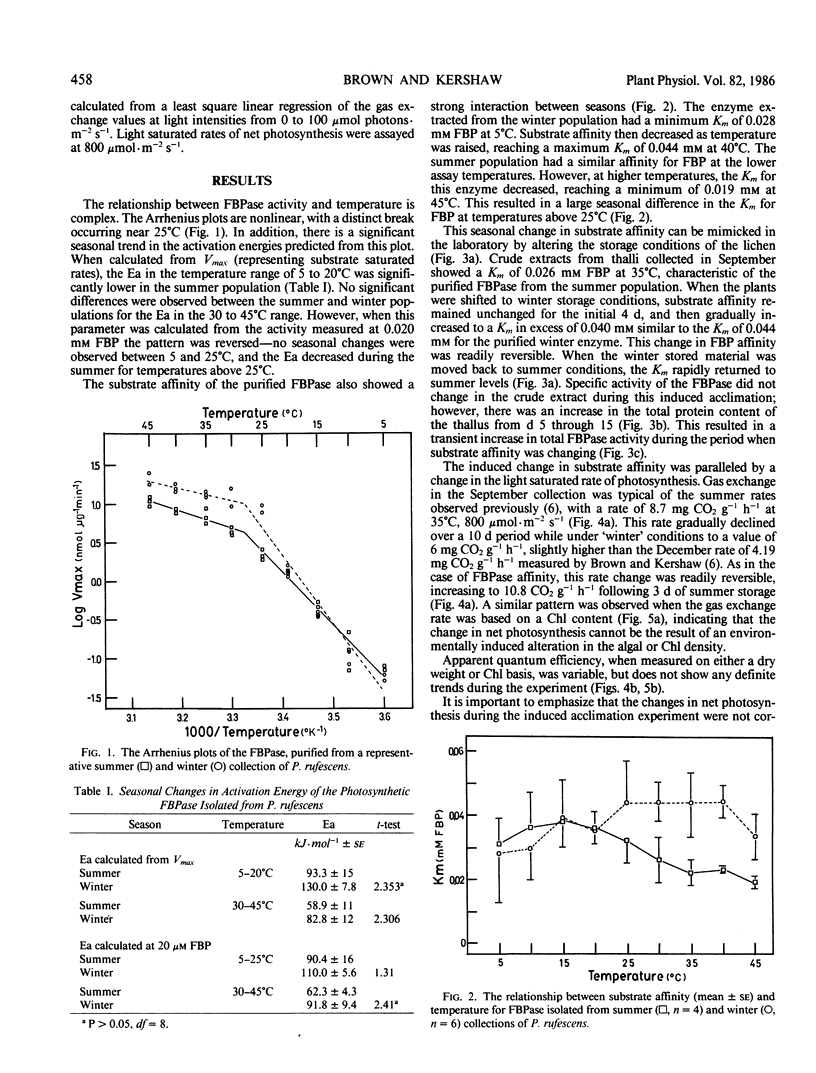
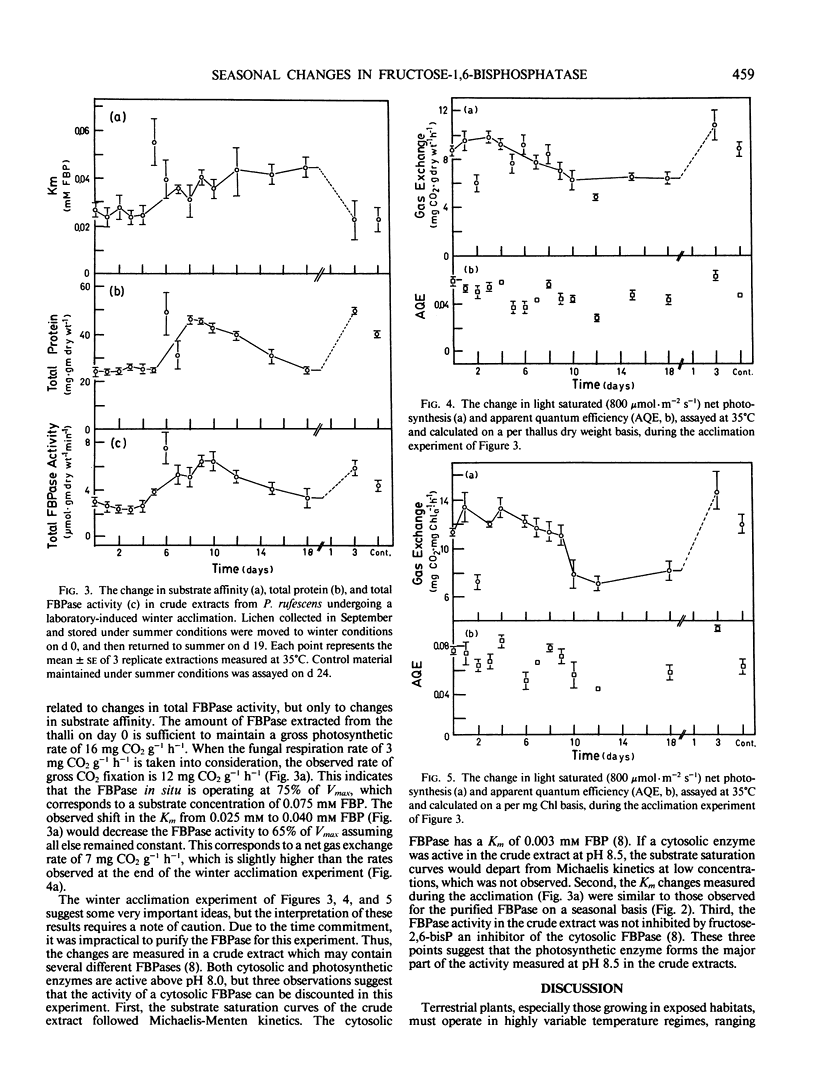
The kinetic parameters of the photosynthetic fructose-1,6-bisphosphatase isolated from Peltigera rufescens (Weis) Mudd. were measured on a seasonal basis and during a laboratory-induced temperature acclimation. Both the substrate affinity and Ea changed on a seasonal basis. During the summer, the Ea decreased from 91.8 to 62.3 kilojoules per mole. The Km fructose-1,6-bisphosphate measured at temperatures above 25°C was also found to decrease by 50%. This seasonal change in Km can be induced by growing the lichen under appropriate conditions for 2 weeks, and is correlated to a change in the net photosynthetic rates. It is hypothesized that this change in fructose-1,6-bisphosphatase is related to the seasonal temperature acclimation process that has been previously reported in this species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J., Hochachka P. W. Functional significance of isoenzymes in thermal acclimatization. Acetylcholinesterase from trout brain. Biochem J. 1970 Mar;116(5):883–887. doi: 10.1042/bj1160883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J., Fairbairn D. Effects of temperature on the kinetics of malate dehydrogenases in the developing eggs and adult muscle of Ascaris lumbricoides (Nematoda). J Exp Zool. 1971 Feb;176(2):169–177. doi: 10.1002/jez.1401760205. [DOI] [PubMed] [Google Scholar]
- Behrisch H. W. Temperature and the regulation of enzyme activity in poikilotherms. Fructose diphosphatase from migrating salmon. Biochem J. 1969 Dec;115(4):687–696. doi: 10.1042/bj1150687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Kershaw K. A. Isolation and Characterization of Two Enzymes Capable of Hydrolyzing Fructose-1,6-Bisphosphatase from the Lichen Peltigera rufescens. Plant Physiol. 1986 Oct;82(2):462–467. doi: 10.1104/pp.82.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Huner N. P., Macdowall F. D. The effects of low temperature acclimation of winter rye on catalytic properties of its ribulose bisphosphate carboxylase-oxygenase. Can J Biochem. 1979 Jul;57(7):1036–1041. doi: 10.1139/o79-130. [DOI] [PubMed] [Google Scholar]
- Low P. S., Bada J. L., Somero G. N. Temperature adaptation of enzymes: roles of the free energy, the enthalpy, and the entropy of activation. Proc Natl Acad Sci U S A. 1973 Feb;70(2):430–432. doi: 10.1073/pnas.70.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero G. N. Temperature as a selective factor in protein evolution: the adaptational strategy of "compromise". J Exp Zool. 1975 Oct;194(1):175–188. doi: 10.1002/jez.1401940111. [DOI] [PubMed] [Google Scholar]
- Waley S. G. An easy method for the determination of initial rates. Biochem J. 1981 Mar 1;193(3):1009–1012. doi: 10.1042/bj1931009. [DOI] [PMC free article] [PubMed] [Google Scholar]


