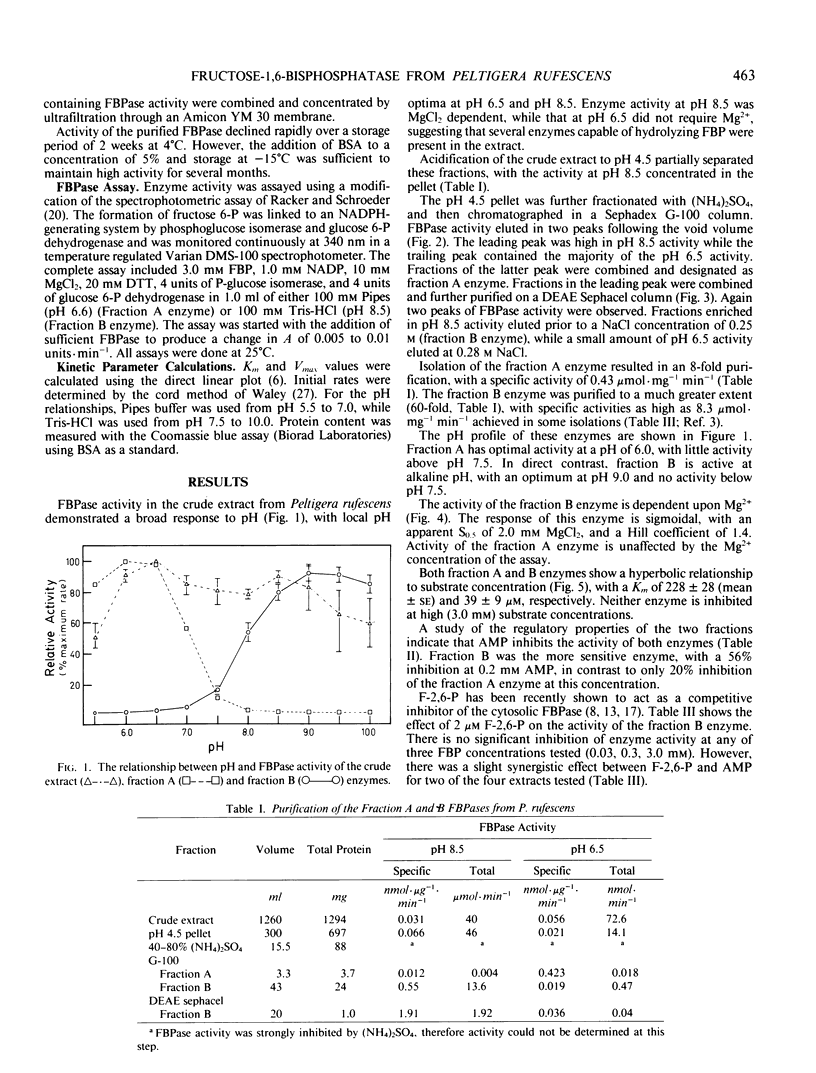
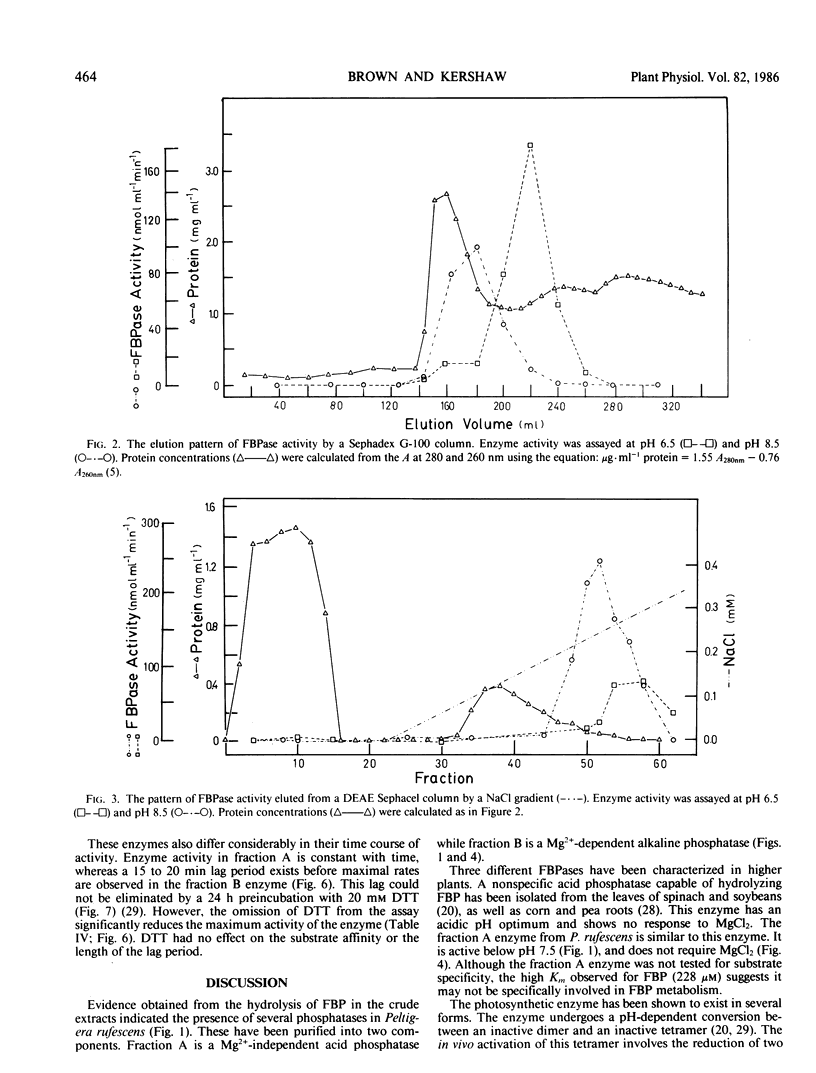
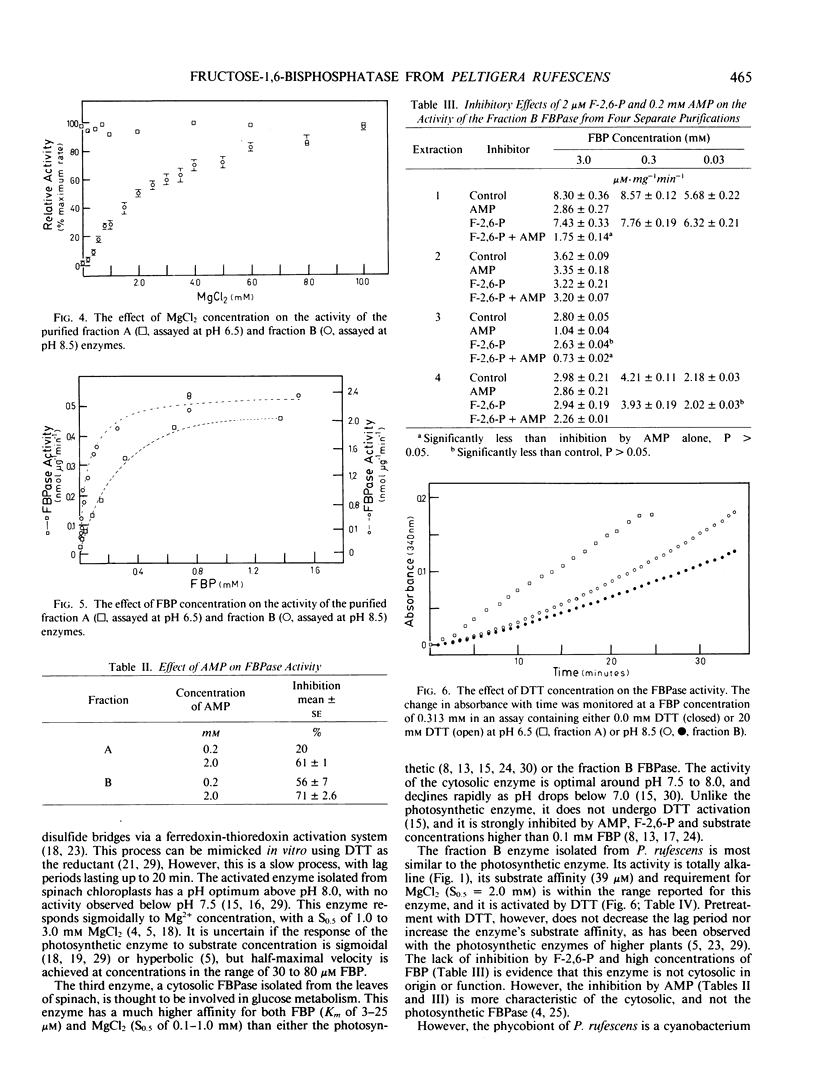
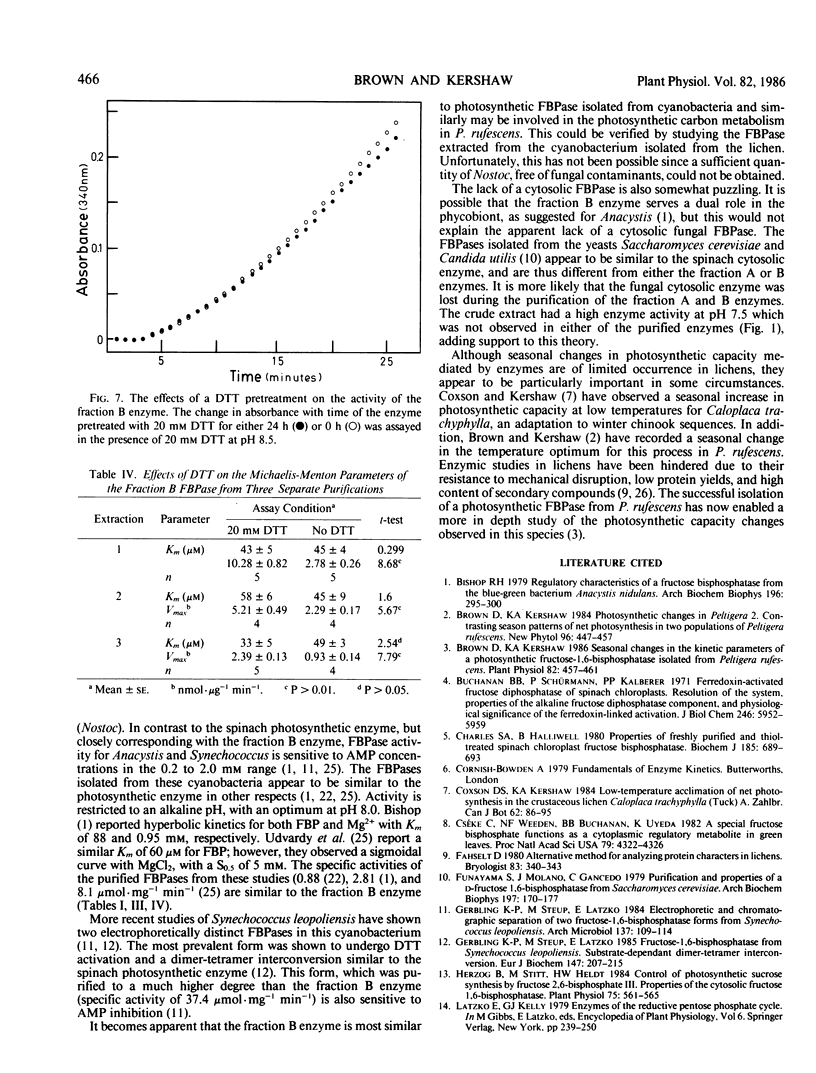
Abstract
Two enzymes capable of hydrolyzing fructose-1,6-bisphosphate (FBP) have been isolated from the foliose lichen Peltigera rufescens (Weis) Mudd. These enzymes can be separated using Sephadex G-100 and DEAE Sephacel chromatography. One enzyme has a pH optimum of 6.5, and a substrate affinity of 228 micromolar FBP. This enzyme does not require MgCl2 for activity, and is inhibited by AMP. The second enzyme has a pH optimum of 9.0, with no activity below pH 7.5. This enzyme responds sigmoidally to Mg2+, with half-saturation concentration of 2.0 millimolar MgCl2, and demonstrates hyperbolic kinetics for FBP (Km = 39 micromolar). This enzyme is activated by 20 millimolar dithiothreitol, is inhibited by AMP, but is not affected by fructose-2-6-bisphosphate. It is hypothesized that the latter enzyme is involved in the photosynthetic process, while the former enzyme is a nonspecific acid phosphatase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R. H. Regulatory characteristics of a fructose bisphosphatase from the blue-green bacterium Anacystis nidulans. Arch Biochem Biophys. 1979 Aug;196(1):295–300. doi: 10.1016/0003-9861(79)90579-4. [DOI] [PubMed] [Google Scholar]
- Brown D., Kershaw K. A. Seasonal Changes in the Kinetic Parameters of a Photosynthetic Fructose-1,6-Bisphosphatase Isolated from Peltigera rufescens. Plant Physiol. 1986 Oct;82(2):457–461. doi: 10.1104/pp.82.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Charles S. A., Halliwell B. Properties of freshly purified and thiol-treated spinach chloroplast fructose bisphosphatase. Biochem J. 1980 Mar 1;185(3):689–693. doi: 10.1042/bj1850689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama S., Molano J., Gancedo C. Purification and properties of a D-fructose 1,6-bisphosphatase from Saccharomyces cerevisiae. Arch Biochem Biophys. 1979 Oct 1;197(1):170–177. doi: 10.1016/0003-9861(79)90233-9. [DOI] [PubMed] [Google Scholar]
- Gerbling K. P., Steup M., Latzko E. Fructose-1,6-bisphosphatase from Synechococcus leopoliensis. Substrate-dependent dimer-tetramer interconversion. Eur J Biochem. 1985 Feb 15;147(1):207–215. doi: 10.1111/j.1432-1033.1985.tb08738.x. [DOI] [PubMed] [Google Scholar]
- Herzog B., Stitt M., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : III. Properties of the Cytosolic Fructose 1,6-Bisphosphatase. Plant Physiol. 1984 Jul;75(3):561–565. doi: 10.1104/pp.75.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Zimmermann G., Feller U. Evidence for a hexosediphosphatase from the cytoplasm of spinach leaves. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):321–326. doi: 10.1515/bchm2.1974.355.1.321. [DOI] [PubMed] [Google Scholar]
- Pradel J., Soulié J. M., Buc J., Meunier J. C., Ricard J. On the activation of fructose-1,6-bisphosphatase of spinach chloroplasts and the regulation of the Calvin cycle. Eur J Biochem. 1981 Jan;113(3):507–511. doi: 10.1111/j.1432-1033.1981.tb05092.x. [DOI] [PubMed] [Google Scholar]
- Preiss J., Biggs M. L., Greenberg E. The effect of magnesium ion concentration on the pH optimum of the spinach leaf alkaline fructose diphosphatase. J Biol Chem. 1967 May 10;242(9):2292–2294. [PubMed] [Google Scholar]
- RACKER E., SCHROEDER E. A. The reductive pentose phosphate cycle. II. Specific C-1 phosphatases for fructose 1,6-diphosphate and sedoheptulose 1,7-diphosphate. Arch Biochem Biophys. 1958 Apr;74(2):326–344. doi: 10.1016/0003-9861(58)90004-3. [DOI] [PubMed] [Google Scholar]
- Rosa L., Whatley F. R. Conditions Required for the Rapid Activation In Vitro of the Chloroplast Fructose-1,6-bisphosphatase. Plant Physiol. 1984 May;75(1):131–137. doi: 10.1104/pp.75.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Herzog B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : V. Modulation of the Spinach Leaf Cytosolic Fructose 1,6-Bisphosphatase Activity in Vitro by Substrate, Products, pH, Magnesium, Fructose 2,6-Bisphosphate, Adenosine Monophosphate, and Dihydroxyacetone Phosphate. Plant Physiol. 1985 Nov;79(3):590–598. doi: 10.1104/pp.79.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardy J., Godeh M. M., Farkas G. L. Regulatory properties of a fructose 1,6-bisphosphatase from the cyanobacterium Anacystis nidulans. J Bacteriol. 1982 Jul;151(1):203–208. doi: 10.1128/jb.151.1.203-208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. An easy method for the determination of initial rates. Biochem J. 1981 Mar 1;193(3):1009–1012. doi: 10.1042/bj1931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Efficient purification and molecular properties of spinach chloroplast fructose 1,6-bisphosphatase. Eur J Biochem. 1976 Nov 15;70(2):361–367. doi: 10.1111/j.1432-1033.1976.tb11025.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann G., Kelly G. J., Latzko E. Purification and properties of spinach leaf cytoplasmic fructose-1,6-bisphosphatase. J Biol Chem. 1978 Sep 10;253(17):5952–5956. [PubMed] [Google Scholar]


