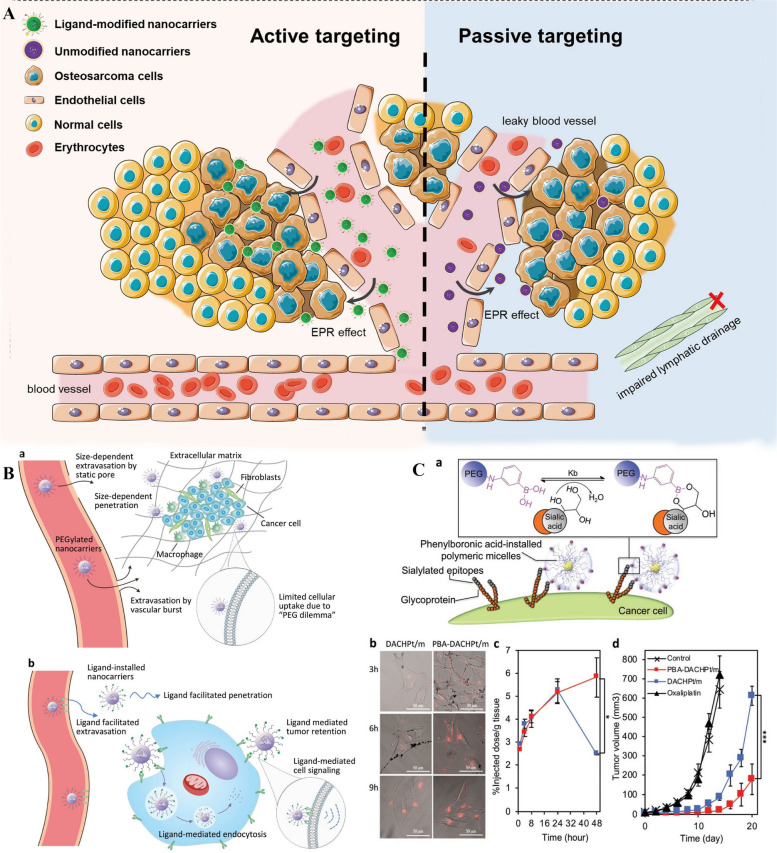
Fig. 1.
A An illustrative diagram depicting the concepts of active and passive targeting in nano-delivery systems for anti-tumor treatment. Passive targeting relies on the enhanced permeability and retention (EPR) effects, where nanocarriers circulate in the bloodstream, exit into the tumor tissue through the leaky tumor blood vessels, and accumulate there. On the other hand, nanocarriers modified with targeting ligands can specifically attach to receptors that are overexpressed on tumor cells, enabling localized drug delivery or internalization via receptor-mediated endocytosis. Reprint from [33] with a permission from Springer Nature. B A diagrammatic representation of a targeting ligand-conjugated nanocarrier. Tumor targeting by nanocarriers (A) and ligand-installed nanocarriers B). Reprint from [34] with a permission from Wiley. C The ability of ligand-installed nanocarriers to target cancer cells. A illustrates the use of phenylboronic-acid-installed DACHPt-loaded polymeric micelles (PBA-DACHPt/m) for targeting cancer cells that overexpress sialylated epitopes receptors; B shows the cellular uptake of micelles with and without PBA ligands by B16F10 cancer cells; C displays the tumor accumulation of PBA-DACHPt/m and DACHPt/m; and (D) exhibits the tumor suppression effect of PBA-DACHPt/m micelles against subcutaneous B16F10 tumor models. Reprint from [34] with a permission from Wiley