Fig. 15.
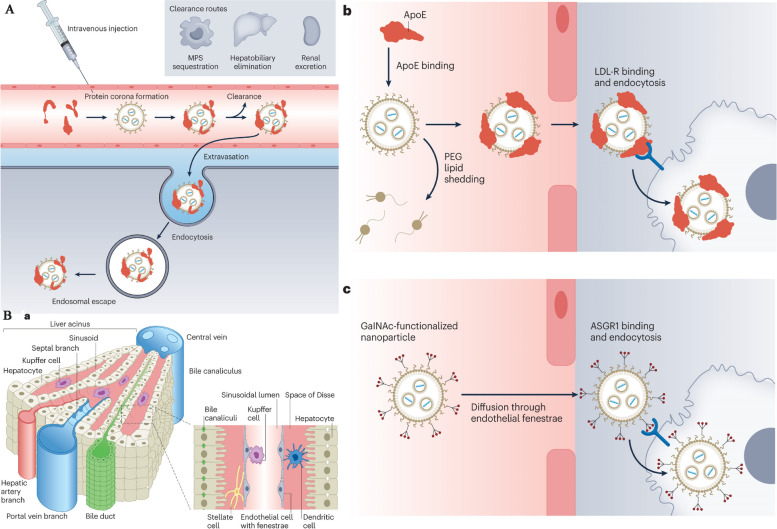
A The path of a tiny particle within the human body after it is injected intravenously. When the particle enters the bloodstream, it often attracts plasma proteins, forming a layer called the protein corona on its surface. The composition of this corona is affected by the properties and makeup of the particle's surface. In order to reach the intended organ, the particle needs to leave the blood vessels (a process known as extravasation) by either passing through gaps in the endothelium (a size-dependent mechanism) or actively interacting with specific receptors on the endothelium through transcytosis. After extravasation, the particle must interact with target cells and be internalized by them. It must then escape from the endosome into the cytosol and release its genetic payload. Throughout this journey, the particle can be eliminated from the bloodstream through various mechanisms such as the mononuclear phagocytic system (MPS), hepatobiliary elimination via feces, or renal excretion through urine. These processes restrict the amount of the injected particle dose that actually reaches the intended target site. Therefore, measures must be taken to minimize their impact. Reprint from [200] with a permission from Springer Nature. B Lipid nanoparticles have reached an advanced stage of development for delivering genetic drugs to the liver. a) The liver consists of four distinct types of cells. When nanoparticles are present in the bloodstream, they can be captured by Kupffer cells, absorbed by liver sinusoidal endothelial cells, or pass through the wide openings in the liver endothelium into the Space of Disse. In the Space of Disse, the nanoparticles can target hepatic stellate cells or hepatocytes. The hepatobiliary system can eliminate nanoparticles from the body through the bile duct. b) A clinically validated approach for delivering small interfering RNA to hepatocytes involves the natural targeting of liver cells. For instance, in the case of Onpattro lipid nanoparticles, the polyethylene glycol (PEG) lipid on the surface of the nanoparticles is exchanged with apolipoprotein E (ApoE) in the blood. The binding of ApoE to the nanoparticle surface enables its interaction with the low-density lipoprotein receptor (LDL-R), which is highly expressed by hepatocytes, leading to endocytosis. c) Another way to actively target hepatocytes is by modifying the nanoparticle surface with a ligand called N-acetylgalactosamine (GalNAc) and reducing non-specific protein binding through extensive PEGylation. GalNAc binds to the asialoglycoprotein receptor 1 (ASGR1), facilitating the uptake of nanoparticles by hepatocytes. Therefore, certain measures need to be taken to minimize their effects. Reprint from [200] with a permission from Springer Nature