Fig. 20.
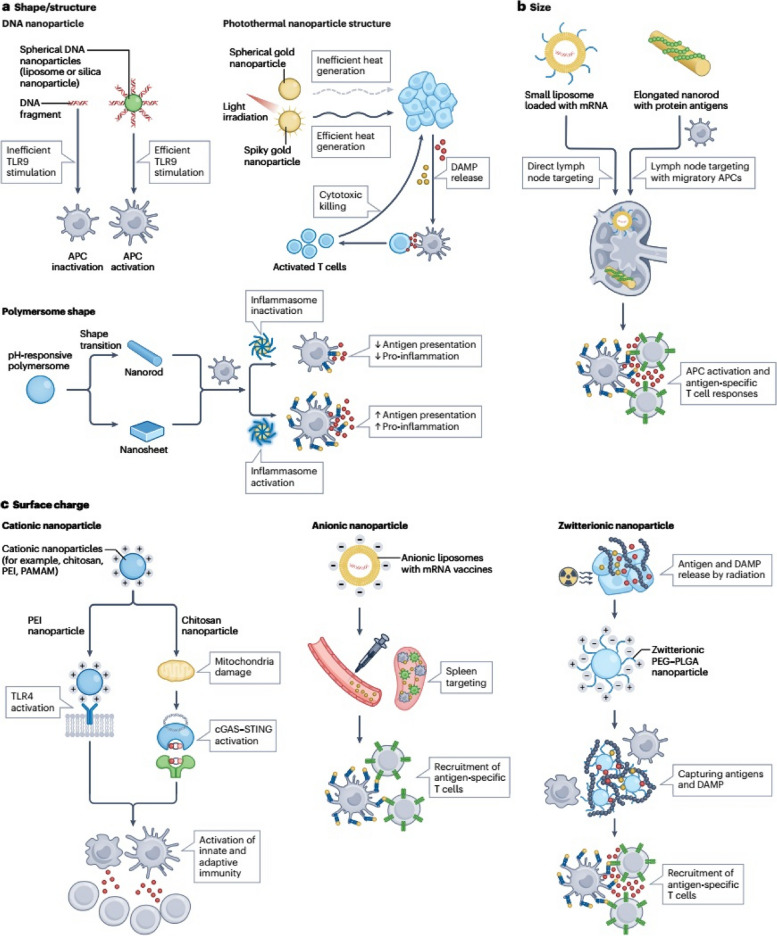
The regulation of immune responses in cancer immunotherapy by nanomaterials' physical properties. A, The shape of nanomaterials can directly or indirectly influence immune responses in innate immune cells. Spherical DNA nanoparticles more effectively stimulate the TLR9 pathway to enhance innate immunity compared to linear DNA fragments. Pointed gold nanoparticles have a higher photothermal efficiency than spherical ones, resulting in greater DAMP release and stronger antitumor immunity. pH-responsive shape transitions from spheres to nanosheets promote inflammasome activation by destabilizing lysosomes, yielding better antitumor immunity than nanorods. The size of organic or inorganic nanomaterials impacts lymph node targeting and nanoparticle retention kinetics, affecting both innate and adaptive immunity for antigen-specific immunogenicity. B, Nanoparticle size influences immunological responses in both innate and adaptive immunity. Adjusting nanoparticle size affects targeting locations; smaller nanoparticles target lymph nodes, while larger ones target antigen-presenting cells (APCs). Large nanoparticles with CD3/CD28 antibodies bind more effectively to T cell receptors than small ones, enhancing T cell immunity. Mesoporous silica nanoparticles with larger pores enable rapid release of immunostimulatory molecules, sensitizing APCs to trigger antitumor responses. C, The surface charge of nanomaterials can directly or indirectly stimulate immune responses. Cationic nanoparticles boost innate immune signaling in APCs, leading to antitumor responses. Anionic nanoparticles, such as mRNA vaccines, when administered systemically, preferentially target the spleen, inducing tumor-specific immunity. Zwitterionic nanoparticles can capture antigens and release DAMPs from dying tumor cells, reprogramming APCs to activate antigen-specific T cell immune responses. Reprint from [130] with a permission from Springer Nature