Abstract
Background
The disability of stroke patients remains an important global health problem; yet information on the extent of restriction from basic and instrumental activities of daily living is limited, particularly in lower-and middle-income (LMIC) countries. Therefore, we examined the issue under the caption, since it is the first step in planning several rehabilitation services.
Method
A facility-based cross-sectional study was done to assess the magnitude and predictors of post-stroke limitations in basic activities of daily living (BADL) using the Barthel Index (BI) scale and instrumental activities of daily living (IADL) using the Frenchay Activities Index (FAI) scale among patients who visited Tikur Anbessa Specialized Hospital in Addis Ababa, Ethiopia, Neurology Clinic from April-October, 2022. All patients having a diagnosis of stroke for more than six months duration were enrolled. Descriptive and inferential statistical analyses were done, and measures of estimated crude and adjusted odds ratio with 95% CI were constructed and a p-value less than 0.05 was considered statistically significant. The results are presented in figures and tables.
Results
A total of 150 stroke patients were enrolled in the present study. The mean age of participants was 53 (14.9) years with slight male preponderance (51.3%). Ischemic stroke was present in 106 (70.7%) of them, while 44 (29.3%) had hemorrhagic stroke. Of this, 57 (38%) and 115 (79.3%) of them had limitations in basic and instrumental ADL, respectively. Comorbid cardiac disease (AOR = 6.9; 95%CI = 1.3–37.5) and regular substance use (AOR = 11.1; 95%CI = 1.1–115) were associated with limitations in BADL, while an increase in age (AOR = 1.1; 95%CI = 1.04–1.15) was associated with severe limitations in BADL. Initial stroke severity (AOR = 7.3; 95%CI = 1.2–44.7) was associated with limitations in IADL, whereas depression (AOR = 5.1; 95%CI = 1.1–23.2) was identified as a predictor of severe limitation in IADL.
Conclusion
Limitation in activities of daily living (ADL) after stroke is common among Ethiopian patients. Therefore, screening for post-stroke limitations in daily activities is essential for further management and rehabilitative plans.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-023-03419-9.
Keywords: Stroke, Activity limitations, Facilities, Disabling factors, Ethiopia
Background
Stroke is regarded as one of the most devastating neurologic disorders affecting around 14 million people worldwide each year [1]. The global burden is shared nationally, with over 52,500 incident cases reported in Ethiopia in the year 2016 [2]. World Stroke Organization (WSO) estimates that over 116 million years of healthy life are lost each year due to stroke-related disability [1]. In one UK study, stroke was associated with the highest odds of severe overall disability, affecting more domains of disability when compared to other disabling conditions like cardiac, mental, and pulmonary diseases [3].
ADL is a concept introduced in 1950 by Sydney Katz. The term collectively describes fundamental skills required to independently care for oneself [4]. It is classified into two types namely basic activities of daily living (BADLs) and instrumental activities of daily living (IADLs). BADL refers to those skills required to manage one’s basic physical needs [4], while IADLs refer to more complex activities related to the ability to live independently in the community [4]. IADLs tend to capture the patient’s ability to live independently in the home and assess a variety of activities like cooking, home management, and recreation. Disability is a long-term physical, mental, intellectual, or sensory impairment that hinders full and effective participation in society on an equal basis with others [5]. Both types of ADL are considered domains of disability and are subject to be affected in individuals with stroke-related motor and cognitive dysfunction.
Previous studies attempted to assess the post-stroke health-related quality of life (HRQOL) [6], in Ethiopia and documented unmet supportive care needs [7] with marked psychological consequences of stroke [8]. On the other hand, anecdotal data are suggestive of high levels of disability among stroke patients [6, 7]; though data on the level of disability, with limitations in basic and instrumental activities of daily living is scanty among stroke survivors in low and middle-income countries (LMIC) including Ethiopia. In view of this, we examined the extent of limitations in basic and instrumental activities of daily living among stroke patients. Moreover, we tried to identify the contributory factors for both basic and instrumental activity limitations and bridge the existing gap of knowledge for future program initiatives.
Methods
Study setting, population, and period
The study was conducted in Tikur Anbessa Specialized Hospital (TASH) from April to October 2022. TASH is located in the capital city of Ethiopia and is the largest government-owned hospital serving as a teaching hospital of Addis Ababa University and a major referral center for the entire country [7]. The hospital provides various services among which the neurology department is considered one of the milestone activities initiated in the country and renders various neurology-related activities such as stroke management. The stroke unit which was recently established operates 24 h, all days of the week and there is a dedicated stroke clinic to treat stroke survivors.
Overview of Acute Stroke Admission and Care at Tikur Anbessa Specialized Hospital (TASH), since the establishment of a Standard of Care Management for Stroke (from November 2021-October 2022)
A total of 141 patients with acute stroke were admitted during an approximate period of one year (from November 2021 to October 2022). Of this, 75 (53.2%) were men. The mean age of admitted patients was 52 years (SD 17.8). Ischemic stroke was diagnosed in 89 (63.1%) of the patients, while the rest 52 (36.9%) had a hemorrhagic stroke. At arrival, the severity of the stroke was assessed using the NIHSS by trained neurology attendings or residents, and results were documented on patients’ charts. As a standard of care, all patients with suspected stroke receive an NIHSS Score at arrival, and this has been fully implemented at our hospital since November 2021. The median (IQR) NIHSS Score of admitted stroke patients during this period was 11 [6, 9]. A total of 124 (87.9%) had a moderate to severe stroke as per the NIHSS, while only 17 (12.1%) had a mild stroke. The inflated number of patients with moderate to severe stroke highlights the fact that TASH offers tertiary-level care for patients across the country.
Study design and participants
A health facility-based cross-sectional study was employed to assess the magnitude and predictors of limitations in activities of daily living among stroke patients that had a follow-up for over six months. The initial follow-up need not be in our specialized hospital and included patients that were referred from other hospitals or transfer-ins. The main eligibility criteria for enrollment of patients were an age of > 18 years and stroke survivors with a stroke duration of more than 6 months. Patients with a stroke duration of less than 6 months were excluded from the study because the tool used to assess IADL is validated for a duration of stroke of 6 months or more [9–11].
The sample size was determined using a single population proportion formula with the assumptions of a 95% level of confidence and a 5% margin of error. Since we could not find any previous studies conducted in Ethiopia to determine limitations in ADL among adult stroke survivors, a proportion value of 50% was used. Assuming a proportion value of 50% would give us the largest sample size to detect a statistically significant difference. Based on these assumptions, a sample size of 384 was obtained. Using a correction formula for a finite population of approximately 200, with a 10% non-response rate, the final sample size was approximated to 150.
A consecutive sampling technique was used to select the participants till the sample size was reached.
Data collection and test procedure
A pretested structured interviewer-administered questionnaire, which contains the socio-demographic, clinical (including depression status), and stroke-related factors was used to collect the data.
BADL was assessed using the Barthel Index (BI), which measures ten basic aspects of self-care and physical dependency (12–13). The 15-item Frenchay Activities Index (FAI), specifically developed for use with stroke patients [9–11], was used in the study to assess IADL.
All data were collected by trained clinicians by relevant guidelines and regulations. Those clients who volunteered to participate in the study were interviewed accordingly.
Data entry, processing, and analysis
Data were entered and analyzed using SPSS version 25 software. Descriptive statistics for the BI and FAI are presented in a table for each item as a proportion. Models of the predictors of activity limitation (both basic (BI < 95) and instrumental (FAI < 30) were developed using binary logistic regression. Bivariate analysis was done to check the existence of crude association and to select candidate variables; those variables which were a-priori clinically important and having (p < 0.25) were included in the final multivariable model. The summary measures of estimated adjusted odd’s ratio (AOR) with 95% CI were constructed and a p-value of less than 0.05 was used to declare statistical significance.
Definitions
Post-stroke Limitations in BADL (assessed by the Barthel Index (BI))
A score of 100 is considered normal, and lower scores indicate increasing disability as per the BI; a BI < 95 corresponds to assisted independence (limitations in BADL) [14], while a BI ≤ 60 corresponds to severe dependency (severe limitation in BADL) [15].
Post-stroke Limitations in IADL (assessed by the Frenchay Activity Index (FAI)): FAI score ranges: from 0(severely restricted) to 45(very active or unrestricted) and can be classified as Scores 31–45(Unrestricted/No limitations in IADLs); FAI score of ≤ 30 defines restriction/limitations in IADL. Scores ranging from 0 − 15 correspond to severe restriction.
Initial stroke severity
National Institute of Health Stroke Scale (NIHSS); defined cut points for mild, moderate, and severe stroke are not well established, but cut-points of NIHSS score as stated may be reasonable; <5 for mild, ≥ 5 for moderate to severe stroke.
Depression: Based on the PHQ-9 validated depression screening tool in general practice settings; a score of < 10: No or minimal Depression, while a Score of 10 or above refers to the presence of Depression [8].
Cognitive Impairment: Mini-mental state exam (MMSE) tool was used to screen for cognitive impairment; 24–30 points: No Cognitive Impairment, ≤ 23: Has Cognitive Impairment.
Modified Rankin Scale (mRS)
Score of.
0- No symptoms at all,
1- No significant disability despite symptoms; able to carry out all usual duties and activities,
2- Slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance,
3- Moderate disability; requiring some help, but able to walk without assistance,
4- Moderately severe disability, unable to walk without assistance and unable to attend to own bodily needs without assistance,
5- Severe disability; bedridden, incontinent, and requiring constant nursing care and attention,
6- Dead.
Regular substance use
refers to as ever using a substance daily or nearly daily for 3 months or more [16]. The substances identified in our study are cigarette, alcohol, and khat (Catha edulis) use.
Results
A total of one hundred and fifty stroke patients on follow-up were included with a response rate of 100%. The mean age was 53(14.9) years and 51.3% were males. 73.3% were married and 79.4% had formal education. 90.0% were urban residents. 56.7% of the patients had no sustainable income and 16.7% used substances regularly (Table 1).
Table 1.
Baseline Socio-demographic characteristics of stroke patients attending TASH, Apr-Oct, 2022
| Socio-demographic Characteristics | Frequency | % | |
|---|---|---|---|
| Age (in Years); Mean (± SD) | 53 (± 14.9) | ||
| Gender | Female | 73 | 48.7 |
| Male | 77 | 51.3 | |
| Marital Status | Married | 110 | 73.3 |
| Never Married | 21 | 14.0 | |
| Divorced | 8 | 5.3 | |
| Widowed | 11 | 7.3 | |
| Residence | Urban | 135 | 90.0 |
| Rural | 15 | 10.0 | |
| Education | No Formal Education | 22 | 14.7 |
| Reading and Writing | 9 | 6.0 | |
| Primary | 25 | 16.7 | |
| Secondary | 48 | 32.0 | |
| More than Secondary | 46 | 30.7 | |
| Employment | Employed | 35 | 23.3 |
| Employed (on paid leave) | 6 | 4.0 | |
| Retired | 24 | 16.0 | |
| Unemployed | 55 | 36.7 | |
| Housewife | 28 | 18.7 | |
| Student | 2 | 1.3 | |
|
Has sustainable Income Status |
Yes | 65 | 43.3 |
| No | 85 | 56.7 | |
| Regular Substance Use * | Yes | 25 | 16.7 |
| No | 125 | 83.3 | |
*identified substances in the study were alcohol, nicotine, and khat (Catha edulis)
Clinical characteristics
As shown in Table 2, Ischemic stroke was diagnosed among 106 (70.7%) stroke survivors. Concerning the time from the last stroke attack, 97 (64.67%) of them had their last stroke attack within the last one year. A history of stroke recurrence was reported among 14 (9.3%) of them. Hypertension and cardiac diseases were the most common medical comorbidities reported among 105 (70%) and 43 (28.7%), respectively. Sixty-five (43.3%) of them screened positive for depressive symptoms (PHQ-9 score of ≥ 10), with the median (IQR) PHQ-9 score being 6 [2–8, 12, 17]. The median (IQR) NIHSS, mRS, and MMSE scores were 10 [5–8, 12, 13, 17, 18], 2 [1–3], and 26 [19–24], respectively; while the median (IQR) duration since the last stroke was 9.5 months (6–36 months).
Table 2.
Clinical Characteristics of Stroke Patients attending TASH, April-Oct, 2022
| Clinical Characteristics | Categories | Number | % |
|---|---|---|---|
| Time Since Last Stroke | ≤ 12 months | 96 | 64.0 |
| > 12 months | 54 | 36.0 | |
| Age at Onset | ≤ 50 years | 74 | 49.3 |
| > 50 years | 76 | 50.7 | |
| Type of Stroke | Ischemic Stroke | 106 | 70.7 |
| Hemorrhagic Stroke | 44 | 29.3 | |
| Area of Stroke | Right Hemisphere | 72 | 48.0 |
| Left Hemisphere | 64 | 42.7 | |
| Both | 7 | 4.6 | |
| Other* | 7 | 4.6 | |
| Aphasia | Yes | 22 | 14.7 |
| No | 128 | 85.3 | |
| Recurrence | Yes | 14 | 9.3 |
| No | 136 | 90.7 | |
| NIHSS Score (N = 97) | Mild | 21 | 21.6 |
| Moderate | 25 | 25.8 | |
| Severe | 51 | 52.6 | |
| MMSE (Adjusted for education) | With Cognitive Impairment | 25 | 16.7 |
| No Cognitive Impairment | 125 | 83.3 | |
| Depression Status | Depression | 65 | 43.3 |
| No Depression | 85 | 56.7 | |
| Hypertension | Yes | 105 | 70.0 |
| No | 45 | 30.0 | |
| Diabetes | Yes | 30 | 20.0 |
| No | 120 | 80.0 | |
| Cardiac Disease | Yes | 43 | 28.7 |
| No | 107 | 71.3 | |
| HIV | Yes | 11 | 7.3 |
| No | 139 | 92.7 |
HIV (Human immunodeficiency virus), MMSE (Mini-mental State Exam), NIHSS (National Institute of Health Stroke Scale), PHQ-9 (Patient Health Questionnaire-9)
* Includes 3 (2%) patients with cerebellar area stroke and 2(1.3%) patients each for the subarachnoid area and venous area strokes. Additional comorbidities include chronic lower back pain (CLBP) in 11 (7.3%) patients, cancer in 5 (3.3%) patients, and peripheral neuropathy in 7 (4.7%) patients
Disability and limitations in activities of daily living
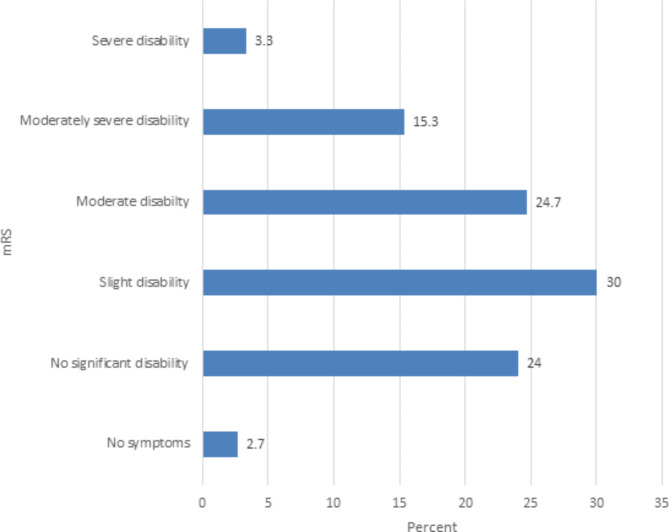
Respondents’ disability measured by the modified Rankin Scale (mRS) revealed moderate to severe disability (mRS of 3 to 5) among 65 (43.3%) of the participants which is denoted in Fig. 1. The level and severity of limitations among stroke survivors based on the BADL and IADL scale measurements are denoted in Table 3.
Fig. 1.
Modified Rankin Scale (mRS) scores of Stroke Patients at TASH, Neurology Clinic, April-October 2022
Table 3.
Level and Severity of Limitations in BADL & IADL of Stroke Patients at TASH, Apr-Oct, 2022
| VariablesCategories | Number% | ||
|---|---|---|---|
| Limitations in BADL (BI < 95) |
With Limitations No Limitations |
57 93 |
38.0% 62.0% |
|
Severe Limitations in BADL (BI ≤ 60) |
Yes | 25 | 16.7% |
| No | 125 | 83.3% | |
| Limitations in IADL (FAI ≤ 30) | With Limitations | 119 | 79.3% |
| No Limitations | 31 | 20.7% | |
| Severe Limitations in IADL (FAI ≤ 15) | Yes | 75 | 50.0% |
| No | 75 | 50.0% | |
ADL (Activities of Daily Living), BADL (Basic Activities of Daily Living), BI (Barthel Index), FAI (Frenchay Activity Index), and IADL (Instrumental Activities of Daily Living)
The median (IQR) BI score was 100 (84–100). The proportion of patients with limitations in basic ADL (dependent or had BI < 95) was 38%, while 79.3% of them had limitations in instrumental ADL (FAI ≤ 30). The median (IQR) FAI score was 16 [6–21, 25–29]. Regarding severity, 16.7% of stroke survivors were severely restricted from basic ADL (BI ≤ 60), while 50% of the study participants were severely restricted from instrumental ADL (FAI ≤ 15).
Factors associated with limitations in activities of daily living among stroke patients included in the study
The factors crudely associated with post-stroke limitations in ADL were assessed using binary logistic regression models. To select the candidate variables of clinical importance, all variables with p < 0.25 were further analyzed in the final multivariable model. Accordingly, regular substance use (AOR = 11.1; 95% CI = 1.1-115.5) and cardiac disease as comorbidity (AOR = 7. 9; 5% CI = 1.3–37.5) were associated with limitations in BADL (as measured by the BI (< 95); while initial stroke severity (NIHSS ≥ 5) (AOR = 7.3; 95% CI = 1.2–44.7) was associated with limitations in IADL (FAI score of < 30).
In the same manner, the factors associated with severe activity limitations were also analyzed (BI ≤ 60 for BADL and FAI ≤ 15 for IADL). An increase in age was associated with severe limitations in BADL (AOR = 1.1; 95% CI = 1.04–1.15), while depression (PHQ9 score of ≥ 10) (AOR = 5.1; 95% CI = 1.1–23.2) was associated with severe restrictions in IADLs (Table 4).
Table 4.
Factors associated with Post-stroke Limitations in Basic and Instrumental Activities of Daily Living in TASH, Apr-Oct, 2022
| % with Limitations in BADL (BI < 95); | % with Severe Limitations in BADL (BI ≤ 60); |
% with Limitations in IADL (FAI ≤ 30); | %with Severe Limitations in IADL (FAI ≤ 15); |
|
|---|---|---|---|---|
| Characteristics | n(%); AOR (95% CI) | n(%); AOR (95% CI) | n(%); AOR (95% CI) | n(%); AOR (95% CI) |
| Age, Mean (SD) | 57 (± 15); | 65(± 14); | 54(± 15); | 57(± 16); |
| 0.985 (0.88,1.1) | 1.1 (1.04–1.15)* | 0.94(0.85,1.03) | 0.99(0.9,1.1) | |
| Duration Since | 17 (31.5); | 20 (20.8); | 81 (84.4); | 54(56.3); |
| Stroke ≤ One Year | 5.18 (0.38,70.5) | 0.000(0.000) | 5.7 (0.007,4834) | 0.84 (0.01,79.7) |
| (N = 96) | ||||
| History of Regular | 16 (64); | 4(16); | 22(88); | 16(64); |
| substance use | 11.1 (1.1,115)* | 0.000(0.000) | 31(1.3,745)* | 3.5 (0.3,38.1) |
| present (N = 25)^ | ||||
| Age at Onset > 50 | 35 (46.1); | 20(26.3); | 67 (88.2); | 45(59.2); |
| years (N = 76) | 5.667 (0.77,45.2) | 0.000(0.000) | 0.249 (0.045,1.4) | 0.29(0.068,1.25) |
| Comorbid Cardiac | 11 (25.6); | 7 (16.3); | 38 (88.4); | 24(55.8); |
| Disease (N = 43) | 6.99 (1.3,37.5)* | 2.1 (0.54,8.02 | 1.9 (0.17,20.1) | 1.9(0.36,10.1) |
| Aphasia (N = 22) | 13 (59.1); | 8(36.4); | 22 (100); | 19(86.4); |
| 3.15 (0.33,29.9) | 0.000(0.000) | 0.000(0.000) | 0.52(0.05,5.24) | |
| Depression | 43 (66.2); | 22(33.8); | 63 (96); | 58(89.2); |
| (N = 65) | 0.58 (0.06,5.3) | 0.000(0.000) | 0.167 (0.01,2.85) | 5.1(1.1,23.2)* |
| Cognitive | 19 (76); | 15 (60); | 25 (100); | 23(92); |
| Impairment | 0.21 (0.15,3.1) | 0.000(0.000) | 0.000(0.000) | 5.8(0.4,83.3 |
| (MMSE ≤ 23) (N = 25) | ||||
| Initial Stroke | 32 (42.1); | 16 (21.1); | 63 (82.9); | 47(61.8); |
| Severity (NIHSS | 0.68 (0.04,10.86) | 0.000(0.000) | 7.33 (1.2,44.7)* | 0.26(0.05,1.43) |
| ≥ 5) (N = 76) |
BADL = Basic Activities of Daily Living; BI = Barthel Index; FAI = Frenchay Activity Index; IADL = Instrumental Activities of Daily Living; MMSE = Minimental State Examination; mRS = Modified Rankin’s Scale; NIHSS = National Institute of Health Stroke Scale; PHQ-9 = Patient Health Questionnaire-9. * represent statistically significant variables with p-values ≤ 0.05. ^Substances of abuse identified in our study include alcohol, nicotine, and khat (Catha edulis). N (capital) represents the number of participants with the mentioned characteristics, while “n”(small) represents the proportion of patients with limitations in ADL.
Discussion
In this study, we sought to identify the level of limitation in basic and instrumental activities of daily living (BADL and IADL respectively) of stroke survivors using the Barthel and Frenchay Activity Indices, respectively. In addition, we further, assessed the contributory factors associated with basic and instrumental ADL.
Overall, the study identified a significant proportion of stroke survivors with activity limitation, in terms of both basic and instrumental ADL (38% and 79.3% respectively) with 16.7% and 50% of them having severe limitations in BADL and IADL, respectively. The data suggests that most of the study participants had some form of disability (73.3%) ranging from slight (mRS of 2) in 30%, to moderate to severe disability (mRS of 3–5) in 43.3% of the participants (Fig. 1). Our findings of post-stroke limitations in BADL are concordant with a prospective Israeli study, which documented a significant rate of limitations in BADL (42.3% of stroke survivors (BI < 95)). This same study revealed the proportion of limitations in IADL (restriction in participation, FAI < 30) to be 28.2% while our finding was 79.3%. The higher proportion of limitations in IADL observed in our study would be related to the number of stroke patients with a stroke duration of less than one year. Lower FAI scores at less than one-year post-stroke were seen in a population-based study, which revealed that 17% of the participants had the lowest possible score of 0 and the average FAI score was 17.4 [29].
Findings similar to our study were also noted in a prospective analysis of the South London Stroke Register which identified between 20% and 30% of stroke survivors to have a poor range of outcomes up to 10 years after stroke [30]. Another study conducted in the Western Cape, South Africa identified moderate to severe limitations in basic ADL (as assessed by the Barthel Index) among 30% of patients after 6 months of stroke onset [31].
Our study identified depression in 43.3% of the study participants. A local study conducted in two centers situated in Addis Ababa, Ethiopia also found a high prevalence of post-stroke depression from a total of 84 ischemic stroke patients (32.2%) [8]. Furthermore, in our study, hypertension was identified as the most common comorbidity (70%), while cardiac comorbidity was identified in 28.7% of the patients. In another study, which assessed the incidence and pattern of stroke among patients admitted to a ward at Yirgalem General Hospital, Southern Ethiopia, similar patterns of comorbidity distribution were identified, whereby hypertension accounted for 71% and cardiac disease (in the form of ischemic or valvular heart disease) accounted for 27.4% [19].
History of regular substance use was reported by 16.6% of participants in our study, which is low when compared with a population-based study in the US which assessed trends in substance abuse preceding stroke among young adults and documented 45–62% of them had substance abuse [20]. The higher numbers seen in the US study could be related to the cohort included in the study which mainly enrolled younger males (56%). Other likely attributes in this regard could be social desirability bias and self-report of substance use, with lower rates of self-report of substance use and underestimation being attested in a study conducted among urban substance users in Baltimore, USA [21]. Nonetheless, the observed findings cannot be overlooked since a significant proportion of regular substance users had limitations in basic (64%) and instrumental (88%) ADLs.
Our study identified regular substance to be associated with BADL, as patients with regular use had higher odds of activity limitations (p-value 0.044). Cardiac disease as a comorbidity was an independent predictor of BADL (p-value 0.023), while initial stroke severity (NIHSS) was a predictor of instrumental ADL (p-value 0.031). Furthermore, an increase in age was found to be associated with severe limitations in BADL (p-value < 0.001), while depression was found to be associated with severe restrictions in IADL (p-value 0.037). In the prospective Israeli study, age at stroke onset and degree of disability were identified as predictors [14], while an Italian study identified impaired mood (depression) as independent predictors of FAI indoor and outdoor activities respectively [22]. In our study, older age at stroke onset was associated with a higher degree of activity limitation, but statistical significance was not met.
Nonetheless, this study was not without its key findings; unlike the studies mentioned above, our study identified cardiac comorbidity and regular substance use as predictors of limitations in BADL. A Serbian study that assessed the impact of comorbidity on rehabilitation outcomes after ischemic stroke identified cardiac illnesses (atrial fibrillation, myocardial infarction, and dilated cardiomyopathy) to be associated with a dismal outcome [23].
In addition, in keeping with prior studies, predictor variables such as employment, type of stroke, location of the stroke, duration since the stroke, stroke recurrence, aphasia, and cognitive impairment were assessed for possible association with ADL, but statistical significance was not met. Employment, type, and location of stroke were not significantly associated with limitations in ADL in the studies conducted in Israel and Italy [14, 22]. Further studies with emphasis to the type of stroke and activity limitation are warranted as the presentation, pathophysiology, and prognosis of small vessel ischemic changes are different from other stroke subtypes [32]. There is a paucity of data when it comes to recurrent stroke and limitations in ADL, as most of the available studies assess only patients with first-ever strokes. In our study, there were merely 14(9.3%) patients with stroke recurrence; hence, a large-scale study is needed to further assess patients with recurrent strokes. Previous studies show that cognitive impairment after a stroke is common and leads to post-stroke dementia. Post-stroke cognitive impairment prevalence varies from 23 to 55% three months after stroke, ending with a decline (11–31%) one year after stroke onset [24, 33, 34]. In this study, 16.7% of the patients had cognitive impairment; although higher rates of activity limitations were seen in patients with cognitive impairment (76% for BADL and 100% for IADL), the findings were not statistically significant. Aphasia was found to be associated with limitations in ADL in a French study that assessed a cohort of 36 aphasic patients [35]. In our study, aphasia was identified in 14.7% of them; higher rates of activity limitation (59.1% for BADL and 100% for IADL) were seen with aphasic patients though not statistically significant. The relatively lower magnitude of aphasia and cognitive impairment, as opposed to the French study in our patient cohort, could contribute to this finding; warranting further large-scale studies.
Strengths and limitations of the study
This study is the first of its kind using a standardized tool for measuring post-stroke limitations in activities of daily living in our setting. In addition, this novel study had a 100% response rate. It further elucidated key factors that need subsequent studies. Nonetheless, the study was not without its limitations. The small sample size used makes it difficult to generalize for the general population. Due to our limited sample size, some variables were clumped together. Hence, dichotomizing variables such as regular substance use may have disproportionately exaggerated our findings. In addition, the use of a non-probability sampling technique might have introduced some bias. Another key source of bias in this hospital-based study is the exclusion of stroke survivors who were unable to attend our stroke clinic, resulting in failure to capture activity limitations in either spectrum of the disease (severely debilitated patients who have defaulted from further follow-up in a tertiary healthcare setting or patients with no significant disability who have been discharged to a primary healthcare setting). Hence, since it is a sample from a tertiary hospital, it may not represent the range of stroke severity at a national level. As with any other cross-sectional study design, the “egg and chicken” dilemma holds true in establishing an etiologic association among variables.
Conclusion and recommendations
In conclusion, limitations in activities of daily living after stroke is a common phenomenon in our setting as seen in other parts of the world. Identification and directed rehabilitative measures for limitations in ADL may enhance patient recovery. Our study demonstrated age, degree of disability, initial stroke severity, regular substance use, cardiac comorbidity and depression to be associated with limitations in ADL. Hence, screening all post-stroke patients to identify both basic and instrumental ADL is essential. Developing a framework capturing ADL as a stroke outcome is key for the proper management of stroke patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all adult stroke survivors who took the time to complete the survey. In addition, the authors express their deepest gratitude to the Department of Neurology and the School of Public Health for their needed level of dedication.
Abbreviations
- AAU
Addis Ababa University
- ADL
Activities of Daily Living
- AOR
Adjusted Odd’s Ratio
- ASA
American Stroke Association
- BADL
Basic Activities of Daily Living
- BI
Barthel Index
- COR
Crude Odd’s Ratio
- FAI
Frenchay Activity Index
- FMOH
Federal Ministry of Health
- IADL
Instrumental Activities of Daily Living
- IBM
International Business Machine
- ICF
International Classification of Functioning
- IQR
Interquartile Range
- HIV
Human Immunodeficiency Virus
- HRQOL
Health-Related Quality of Life
- LMIC
Low and Middle-Income Countries
- MMSE
Mini-mental Status Exam
- mRS
modified Rankin Scale
- NIHSS
National Institute of Health Stroke Scale
- OR
Odd’s Ratio
- PHQ
9-Patient Health Questionnaire-9
- SPSS
Statistical Package for Social Sciences
- TASH
Tikur Anbessa Specialized Hospital
- UNCRPD
The United Nations Convention on the Rights of Persons with Disabilities
- USA
United States of America
- WHO
World Health Organization
- WSO
World Stroke Organization
Author contributions
Dr. Salhadin Mohammed was responsible for project inception, data analysis, and drafting of the main manuscript; while Dr. Yared Mamushet Yifru and Dr. Biniyam Alemayehu Ayele supervised the work. Professor Jemal Haidar critically reviewed the manuscript for the intellect. All authors read and approved the final manuscript.
Funding
The authors report no external funding source for this study.
Data Availability
All data generated or analyzed are included in this published article.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. The study was approved by the Ethics Committee of the Department of Neurology, School of Medicine, Addis Ababa University, and verbal informed consent was obtained from each study participant which was approved by the Ethics Committee of Department of Neurology, School of Medicine, Addis Ababa University. Study participants identified to have depression, severe limitations in ADL, and/or aphasia were linked to the appropriate units and clinics.
Consent for publication
Not Applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.M Patrice Lindsay (Corresponding Author)1 BNRLSMBWHSMJPVF. Global Stroke Fact Sheet. 2019. World Stroke Organ [Internet]. 2019; Available from: https://www.world-stroke.org/assets/downloads/WSO_Fact-sheet_15.01.2020.pdf.
- 2.GBD 2016 Stroke Collaborators. 10.1016/S1474-4422(19)30034-1. Lancet Neurol. 2019;18(5):439–58. Epub 2019 Mar 11. PMID: 30871944; PMCID: PMC6494974. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. [DOI] [PMC free article] [PubMed]
- 3.Adamson J, Beswick A, Ebrahim S. Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis. 2004 Jul-Aug;13(4):171-7. 10.1016/j.jstrokecerebrovasdis.2004.06.003. PMID: 17903971. [DOI] [PubMed]
- 4.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–7. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 5.Nations U. Convention on the Rights of Persons with Disabilities and Optional Protocol. Available from https://www.un.org/development/desa/disabilities/convention-on-the-rights-of-persons-with-disabilities.html#:~:text=The%20Convention%20on%20the%20Rights%20of%20Persons%20with,was%20opened%20for%20signature%20on%2030%20March%202007.
- 6.Zemed A, Chala KN, Eriku GA, Aschalew AY. Health-related quality of life and associated factors among patients with stroke at tertiary level hospitals in Ethiopia. PLoS One [Internet]. 2021;16(3 March):1–15. 10.1371/journal.pone.0248481. [DOI] [PMC free article] [PubMed]
- 7.Tamrat EG, Gufue ZH, Getachew S, Yifru YM, Gizaw M. Factors associated with the longer-term unmet supportive care needs of stroke survivors in Ethiopia: a multicentre cross-sectional study. BMJ Open. 2022;12(1):e053579. doi: 10.1136/bmjopen-2021-053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fikru Tsehayneh A, Tafesse. “High Prevalence of Poststroke Depression in Ischemic Stroke Patients in Ethiopia”, Neurology Research International, vol. 2020, Article ID 8834299, 6 pages, 2020. 10.1155/2020/8834299. [DOI] [PMC free article] [PubMed]
- 9.Schuling J, de Haan R, Limburg M, Groenier KH. The Frenchay Activities Index. Assessment of functional status in stroke patients. Stroke. 1993;24(8):1173-7. 10.1161/01.str.24.8.1173. PMID: 8342192. [DOI] [PubMed]
- 10.Post MW, de Witte LP. Good inter-rater reliability of the Frenchay Activities Index in stroke patients. Clin Rehabil. 2003;17(5):548 – 52. 10.1191/0269215503cr648oa. PMID: 12952162. [DOI] [PubMed]
- 11.Tooth LR, McKenna KT, Smith M, O’Rourke P. Further evidence for the agreement between patients with stroke and their proxies on the Frenchay Activities Index. Clin Rehabil. 2003;17(6):656 – 65. 10.1191/0269215503cr661oa. PMID: 12971711. [DOI] [PubMed]
- 12.Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke. 2011;42(4):1146-51. 10.1161/STROKEAHA.110.598540. Epub 2011 Mar 3. PMID: 21372310. [DOI] [PubMed]
- 13.Duffy L, Gajree S, Langhorne P, Stott DJ, Quinn TJ. Reliability (inter-rater agreement) of the Barthel Index for assessment of stroke survivors: systematic review and meta-analysis. Stroke. 2013;44(2):462–8. doi: 10.1161/STROKEAHA.112.678615. [DOI] [PubMed] [Google Scholar]
- 14.Gadidi V, Katz-Leurer M, Carmeli E, Bornstein NM. Long-term outcome poststroke: predictors of activity limitation and participation restriction. Arch Phys Med Rehabil. 2011;92(11):1802-8. 10.1016/j.apmr.2011.06.014. PMID: 22032214. [DOI] [PubMed]
- 15.Carod-Artal J, Egido JA, González JL, Varela de Seijas E. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke. 2000;31(12):2995–3000. 10.1161/01.str.31.12.2995. PMID: 11108762. [DOI] [PubMed]
- 16.Harrell PT, Trenz RC, Scherer M, Pacek LR, Latimer WW. Cigarette smoking, illicit drug use, and routes of administration among heroin and cocaine users. Addict Behav. 2012;37(5):678–81. doi: 10.1016/j.addbeh.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO_HSC_ACE_99.2.pdf. Available from https://apps.who.int/iris/bitstream/handle/10665/65990/WHO_HSC_ACE_99.2.pdf Retrieved March 2, 2022.
- 18.Saver JL, Johnston KC, Homer D, Wityk R, Koroshetz W, Truskowski LL, Haley EC. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS Investigators. Stroke. 1999;30(2):293-8. 10.1161/01.str.30.2.293. PMID: 9933262. [DOI] [PubMed]
- 19.Agazhe M, Eshetu D, Arsicha A, et al. Incidence and pattern of stroke among patients admitted to medical ward at Yirgalem General Hospital, Sidama Regional State, Southern-Ethiopia. SAGE Open Medicine. 2021;9:20503121211001154. doi: 10.1177/20503121211001154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De los Ríos F, Kleindorfer DO, Khoury J, Broderick JP, Moomaw CJ, Adeoye O, Flaherty ML, Khatri P, Woo D, Alwell K, Eilerman J, Ferioli S, Kissela BM. Trends in substance abuse preceding stroke among young adults: a population-based study. Stroke. 2012;43(12):3179–83. doi: 10.1161/STROKEAHA.112.667808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latkin CA, Edwards C, Davey-Rothwell MA, Tobin KE. The relationship between social desirability bias and self-reports of health, substance use, and social network factors among urban substance users in Baltimore, Maryland. Addict Behav. 2017;73:133–136. 10.1016/j.addbeh.2017.05.005. Epub 2017 May 9. PMID: 28511097; PMCID: PMC5519338. [DOI] [PMC free article] [PubMed]
- 22.Andrenelli E, Ippoliti E, Coccia M, Millevolte M, Cicconi B, Latini L, Lagalla G, Provinciali L, Ceravolo MG, Capecci M. Features and predictors of activity limitations and participation restriction 2 years after intensive rehabilitation following first-ever stroke. Eur J Phys Rehabil Med. 2015;51(5):575–85. [PubMed] [Google Scholar]
- 23.Simić-Panić D, Bošković K, Milićević M, Rabi Žikić T, Cvjetković Bošnjak M, Tomašević-Todorović S, Jovićević M. The impact of Comorbidity on Rehabilitation Outcome after ischemic stroke. Acta Clin Croat. 2018;57(1):5–15. doi: 10.20471/acc.2018.57.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Qazzaz NK, Ali SH, Ahmad SA, Islam S, Mohamad K. Cognitive impairment and memory dysfunction after a stroke diagnosis: a post-stroke memory assessment. Neuropsychiatr Dis Treat. 2014;10:1677–91. doi: 10.2147/NDT.S67184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiemanck SK, Post MW, Witkamp TD, Kappelle LJ, Prevo AJ. Relationship between ischemic lesion volume and functional status in the 2nd week after middle cerebral artery stroke. Neurorehabil Neural Repair. 2005;19(2):133-8. 10.1177/154596830501900207. PMID: 15883357. [DOI] [PubMed]
- 26.Pan SL, Wu SC, Lee TK, Chen TH. Reduction of disability after stroke is a more informative predictor of long-time survival than initial disability status. Disabil Rehabil. 2007;29(5):417 – 23. doi: 10.1080/09638280600836042. PMID: 17364795. [DOI] [PubMed]
- 27.Huybrechts KF, Caro JJ. The Barthel Index and modified Rankin Scale as prognostic tools for long-term outcomes after stroke: a qualitative review of the literature. Curr Med Res Opin. 2007;23(7):1627-36. 10.1185/030079907x210444. PMID: 17559756. [DOI] [PubMed]
- 28.Murray CD, Harrison B. The meaning and experience of being a stroke survivor: an interpretative phenomenological analysis. Disabil Rehabil. 2004;26(13):808 – 16. 10.1080/09638280410001696746. PMID: 15371053. [DOI] [PubMed]
- 29.Appelros P. Characteristics of the Frenchay Activities Index one year after a stroke: a population-based study. Disabil Rehabil. 2007;29(10):785 – 90. 10.1080/09638280600919715. PMID: 17457736. [DOI] [PubMed]
- 30.Wolfe CD, Crichton SL, Heuschmann PU, McKevitt CJ, Toschke AM, Grieve AP, Rudd AG. Estimates of outcomes up to ten years after stroke: analysis from the prospective South London Stroke Register. PLoS Med. 2011;8(5):e1001033. doi: 10.1371/journal.pmed.1001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhoda A, Mpofu R, Weerdt W. Activity limitations of patients with stroke attending out-patient facilities in the Western Cape, South Africa. South Afr J Physiotherapy. 2011;67. 10.4102/sajp.v67i2.41.
- 32.Rudilosso S, Rodríguez-Vázquez A, Urra X, Arboix A. The potential impact of Neuroimaging and Translational Research on the Clinical Management of Lacunar Stroke. Int J Mol Sci. 2022;23:1497. doi: 10.3390/ijms23031497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8(1):38–45. 10.1111/j.1747-4949.2012.00972.x. PMID: 23280268. [DOI] [PubMed]
- 34.Snaphaan L, de Leeuw FE. Poststroke memory function in nondemented patients: a systematic review on frequency and neuroimaging correlates. Stroke. 2007;38(1):198–203. doi: 10.1161/01.STR.0000251842.34322.8f. [DOI] [PubMed] [Google Scholar]
- 35.Darrigrand B, Dutheil S, Michelet V, Rereau S, Rousseaux M, Mazaux JM. Communication impairment and activity limitation in stroke patients with severe aphasia. Disabil Rehabil. 2011;33(13–14):1169–78. doi: 10.3109/09638288.2010.524271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed are included in this published article.