Figure 2.
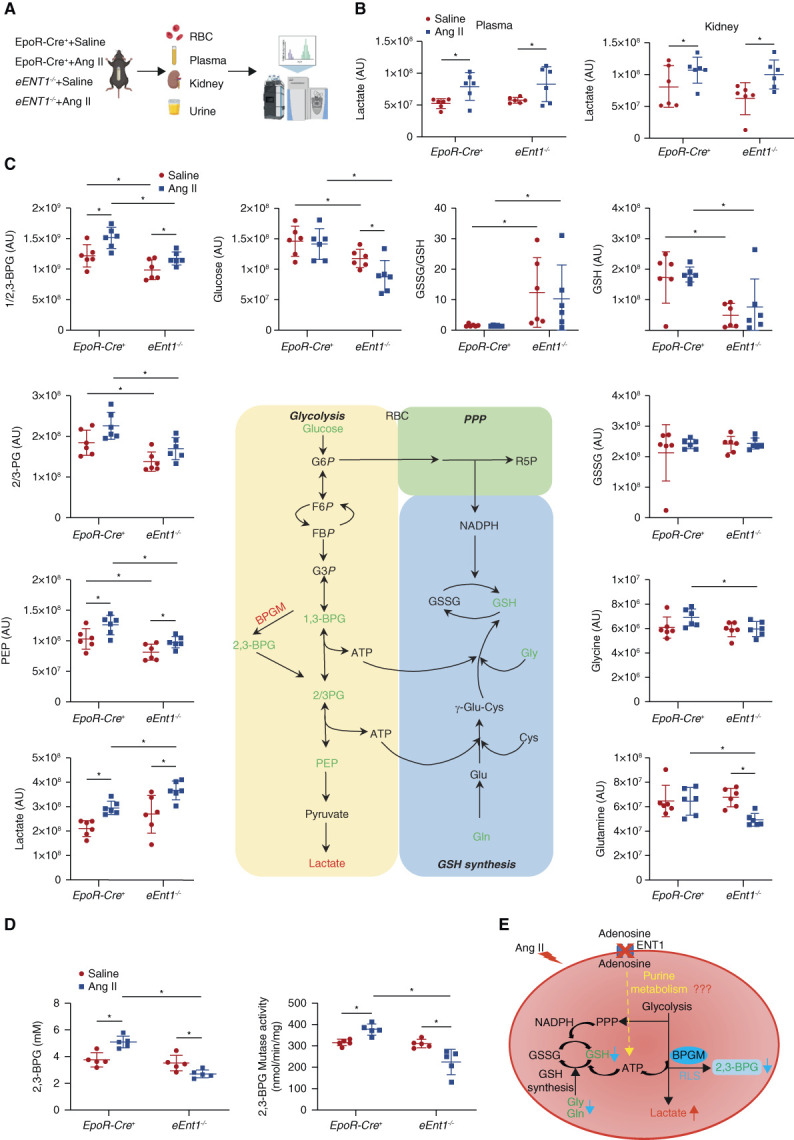
eENT1 mitigates severe hypoxia-induced renal oxidative stress and damage by enhancing erythrocyte BPGM activation and 2,3-BPG production. (A) Experimental design for mouse sample collection and metabolomics study. (B) Plasma and kidney lactate levels in metabolomics screening. Data are expressed as mean±SEM. N=6 for each group. P values were assessed using the ordinary one-way ANOVA with the Holm–Sidak post hoc multiple comparisons test. (C) Erythrocyte intermediates of glycolysis (glucose, 2,3-bisphosphoglycerate, 2/3-phosphoglycerate, phosphoenolpyruvate, and lactate), PPP, and glutathione synthesis (glycine, glutamine, GSH, and GSSG) were revealed by untargeted metabolomics. Data are expressed as mean±SEM. N=6 for each group. P value was assessed using the ordinary one-way ANOVA with the Holm–Sidak post hoc multiple comparisons test. *P < 0.05. (D) Erythrocyte 2,3-BPG levels and BPGM activity were measured using commercial kit. Data were expressed as mean±SEM. N=5 mice in each group. P values were assessed using the ordinary one-way ANOVA with the Holm–Sidak post hoc multiple comparisons test. *P < 0.05. (E) Proposed mechanism: loss of adenosine uptake in eEnt1−/− mice impairs purine metabolism, leading to reduced BPGM activity and 2,3-BPG production. Figure 2 can be viewed in color online at www.jasn.org.
