Abstract
Objectives
Social stress has been shown to affect immune functioning. Past research has found that chronic social stress and latent viral infections accelerate immune aging, leading to chronic disease morbidity and mortality. Chronic stress may also reactivate latent viral infections, like cytomegalovirus (CMV), accelerating the aging of the immune system.
Method
Utilizing panel survey data from 8,995 U.S. adults aged 56 or older from the Health and Retirement Study, this study investigates whether chronic stress interacts with CMV positivity to drive aging of the immune system, multimorbidity, and mortality.
Results
Results of moderated mediation analysis indicate that the effect of CMV positivity on morbidity and mortality as mediated by immune aging indicators is amplified by chronic stress.
Discussion
These findings suggest that immune aging is a biological pathway underlying the stress process and help explain past findings in the literature on stress and health.
Keywords: Cytomegalovirus, Health and Retirement Study, Immunosenescence
Recent viral outbreaks, including coronavirus disease 2019 (COVID-19) and mpox, have highlighted the importance of viral infections and immune functioning for health and well-being (Mahase, 2022). It is increasingly clear both exposure to viruses and the consequences of exposure are socially structured and are influenced by social forces (Millett et al., 2020). Exposure to social stressors, experiences related to social position, relationships, and experiences that are challenging or difficult (e.g., chronic financial strain, conflict at work, and caretaking for a sick family member), has been associated with accelerating the aging of the immune system, leading to poorer response to viruses and other infections, poorer response to vaccines, chronic disease morbidity, and mortality (Hayward et al., 2020; Klopack, Crimmins, et al., 2022). Social stress may also reactivate latent viral infections, amplifying the health hazard associated with viral infection (Noppert et al., 2021; Reed et al., 2019).
One such virus is human cytomegalovirus (CMV), a ubiquitous herpesvirus. Past research has shown that CMV positivity is associated with poorer immune functioning, leading to accelerated aging and greater morbidity and mortality (Chen et al., 2021; Ford et al., 2020; Kananen et al., 2015; Pawelec et al., 2012). Like other herpesviruses, CMV is not fully cleared after initial infection, but remains latent, periodically reactivating. Thus, the immune system must commit substantial resources to managing CMV infections. Research suggests social stress may reactivate CMV, accelerating the aging of the immune system (Elwenspoek et al., 2017; Reed et al., 2019). CMV is more prevalent in disadvantaged populations, including people in lower socioeconomic positions, Black and Hispanic Americans, and gender and sexual minorities (Cannon et al., 2010; Dowd et al., 2009; Hoes et al., 2018; Noppert et al., 2021).
To understand how social stress, immune functioning, and viral infection interact to affect health, I focus on immune aging. Immune aging, or immunosenescence, refers to changes in the composition of immune cells associated with age. Immune aging is associated with a number of important health outcomes, including chronic disease morbidity and mortality (Broadley et al., 2017; Huff et al., 2019), and emerging evidence suggests that social stress accelerates immune aging (Klopack, Crimmins, et al., 2022; Noppert et al., 2021; Prather et al., 2018). Thus, immune aging appears to play an important role in the relationship between stress and health.
To analyze these arguments, I utilize data from 8,995 U.S. adults aged 56 or older from the Health and Retirement Study (HRS). These data represent the first time that such detailed immunotyping has been conducted in a large nationally representative sample of older adults, presenting a unique opportunity to assess the social determinants of immune aging and address novel questions about how changes in the immune system may help explain the stress and health process.
Social Stress and Immune Aging
Immune aging
I focus on age-related changes in the adaptive immune system—so-called immune aging or immunosenescence—in the current study. In past research, stress has been associated with a repressed ability to respond to immunological insults, including bacteria and viruses like COVID-19 (Evans, 2020; Glaser & Kiecolt-Glaser, 1997; Segerstrom & Miller, 2004). This is because stress appears to affect the functioning of both the innate and adaptive immune systems (Fulop et al., 2018). The innate immune system is the so-called first line of immunological defense. This system includes basic pattern-recognition systems to respond immediately to injury and immunological insult as well as inflammatory signaling to prevent the spread of threats. The adaptive immune system involves a more sophisticated specific pattern-recognition system and is needed for robust responses to new threats, as well as developing memory for better protection in the future.
Adaptive immune aging may provide a window into biological processes underlying the association between stress and health. Immune aging refers to changes in the immune system, including lower percentages of naïve T lymphocytes (Aiello et al., 2019). Naïve T cells have not been presented with an antigen and are needed to respond to novel antigens (e.g., novel viruses like COVID-19) and to vaccines. As people age, their thymus shrinks and is replaced by fatty tissue—or involutes. Because new naïve T cells mature in the thymus, thymic involution leads to fewer naïve T cells and an accumulation of memory and senescent-like T cells (Pangrazzi & Weinberger, 2020). Thus, a higher proportion of naïve T cells relative to memory, effector, and other late-differentiated T cells is indicative of less immune aging (Aiello et al., 2019; Klopack, Crimmins, et al., 2022). Immune aging has been linked to cardiovascular disease, cancer, and other chronic conditions as well as reduced efficacy in fighting acute infections and reduced response to vaccines (Aiello et al., 2019; Thyagarajan et al., 2022). Past research suggests that naïve T lymphocytes are particularly affected by immune aging (Pangrazzi & Weinberger, 2020; Prather et al., 2018) and have been used in past research as important indicators of immune aging (Aronoff et al., 2022; Klopack, Crimmins, et al., 2022; Prather et al., 2018; Ramasubramanian et al., 2022; Thyagarajan et al., 2022). They are therefore the focus of the current study.
T cells can be divided into helper (CD4+), which assist and direct the activity of other lymphocytes, and cytotoxic (CD8+) cells, which target and destroy infected cells and cancerous cells. Age-related decline in the percentage of naïve CD8+ T cells typically happens earlier in life, whereas CD4+ T cell tend to be more stable (Arnold et al., 2011; Pangrazzi & Weinberger, 2020). CD8+ T cells may, therefore, be more sensitive to exposures like CMV and chronic stress.
Stress and immune aging
Past research suggests that ongoing chronic stressors are strongly associated with poorer health (Crielaard et al., 2021; Lantz et al., 2005; Pearlin, 2010; Serido et al., 2004), and emerging evidence suggests that immune aging is accelerated by social stressors (Aiello, Feinstein, et al., 2016; Klopack, Crimmins, et al., 2022; Noppert et al., 2021). The hypothalamic–pituitary–adrenal axis and sympathetic nervous system are activated in response to stress, potentially help to prepare an individual to deal with an immediate threat or injury (Cole, 2019; McEwen & Seeman, 1999; McEwen & McEwen, 2017). However, when people experience chronic and ongoing stress, these stress response systems can be chronically overactivated, leading to wear and tear and systemic inflammation (McEwen & McEwen, 2017) and potentially accelerated thymic involution (de Felice et al., 2008). Additionally, people who experience more stress tend to engage in health-risk behaviors as a way of coping. These behaviors, including smoking, drinking alcohol, poor diet, and low exercise, are associated with thymic involution (Duggal et al., 2018; Spadaro et al., 2022). Stress also accelerates cellular and biological aging, which may, in turn, drive immune aging (Aiello, Dowd, et al., 2016; Aronoff et al., 2022; Needham et al., 2013; Simons et al., 2021). Thus, immune aging may be a biological pathway by which stress affects morbidity and mortality.
Human cytomegalovirus
In the current study, I focus on CMV infection, which has been shown to have substantial effects on immune cell composition and has been shown to accelerate immune aging (Kananen et al., 2015; Klopack, Crimmins, et al., 2022). CMV is a herpesvirus that is common throughout human populations. Following primary infection, CMV remains latent (i.e., is never fully cleared) and can reactivate across the life course. CMV reactivation is associated with social stress (Reed et al., 2019). Thus, experiencing stress in later life may interact with CMV infection acquired earlier in life. The immune system must dedicate large amounts of resources to keep CMV in check, leading to more naïve and memory cells committed to the virus (so-called memory inflation; Solana et al. 2012).
Structured exposure to CMV
Because of environmental, structural, systemic, and interpersonal racism, people of color in the United States experience greater CMV exposure. Racialized groups in the United States tend to occupy lower socioeconomic status (SES) positions, tend to live in more disadvantaged neighborhoods, experience more congenital transmission, experience more housing crowding, and tend to live in states in the United States with higher CMV seroprevalence, all of which increase the risk of CMV (Cannon et al., 2010; Colugnati et al., 2007; Hotez, 2008; Staras et al., 2006). Other structured inequalities are associated with greater viral burden, including SES which is associated with housing crowding, larger family size, less sanitary living conditions, more risky sexual behavior, and greater daycare utilization, all of which are associated with greater CMV exposure (Cannon et al., 2010; Dowd et al., 2009; Hoes et al., 2018).
Current Study
In the current study, I investigated (1) whether adult chronic stress exposure is associated with immune aging, morbidity, and mortality; (2) whether immune aging mediates the association between chronic stress and morbidity and mortality; and (3) whether chronic stress interacts with CMV infection to accelerate immune aging and increase risk of morbidity and mortality. I utilize newly released data from the HRS, a large, nationally representative sample of older U.S. adults. Though there is evidence for the model described above in past research, much of this research has only examined a single pathway at a time and has utilized small, nonrepresentative, or specialty samples (e.g., patients undergoing cancer treatment and caregivers, see e.g., Prather et al., 2018).
Method
Sample
I utilize data from the HRS 2016 Venous Blood Study (VBS; N = 9,934; Health and Retirement Study, 2021). As part of the 2016 data collection, venous blood was drawn from consenting respondents who participated in an in-home interview. A certified phlebotomist collected the blood within four weeks of the HRS interview when possible. Fasting was preferred, but not required. When weighted, this sample was designed to be representative of community-dwelling adults aged 56 or older in the United States. In addition to these biological data, I utilized survey data collected in 2014, 2016, 2018, and 2020 for the current study. For more information see Crimmins et al. (2017).
Measures
Mortality
Mortality was assessed as people known to be deceased by HRS in 2020.
Multimorbidity
Respondents were asked if a doctor had ever told them they had a number of conditions, including “high blood pressure or hypertension,” “diabetes or high blood sugar,” “cancer or a malignant tumor of any kind except skin cancer,” “chronic lung disease except asthma such as chronic bronchitis or emphysema,” “heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems,” “stroke or transient ischemic attack,” or “arthritis or rheumatism.” Affirmative responses from 2016 were summed to create a multimorbidity index. If responses were missing in 2016, 2014 responses were used.
Immune aging
Flow cytometry was used to assess percentages of 24 immune cells using the standardized protocol by the Human Immunology Project (Maecker et al., 2012) with minor modifications performed on an LSRII or a Fortessa X20 flow cytometer (BD Biosciences, San Diego, CA) More detailed methods are available elsewhere (Crimmins et al., 2017). I focus on the percentage of naïve CD4+ (CD4+/CD3+/CD19−/CD45RA+/CCR7+/CD28+) and CD8+ T cells (CD8+/CD3+/CD19−/CD45RA+/CCR7+/CD28+). These percentages are in proportion to the total number of CD4+ and CD8+ T cells, respectively. That is, these measures indicate the relative proportion of naïve cells in these compartments compared with effector, memory, and other late differentiated cells. Having more naïve relative to other cell types is an indicator of a less aged immune system.
Chronic stress
Chronic stress was assessed using an eight-item scale (Troxel et al., 2003) that includes both the number of ongoing stressful problems and how distressing these problems are. Sample items included “alcohol or drug use in family member,” “financial strain,” and “housing problems” with responses ranging from 1 (no, didn’t happen) to 4 (yes, very upsetting). The eight items were averaged together to create this scale. This questionnaire is given to a rotating random half of the core panel every other wave so that data are available for the full sample every four years. I use data from the 2014 and 2016 leave-behind questionnaires. Because this variable was included in an interaction term, I mean centered it.
CMV seropositivity
IgG antibodies assessed in serum with the Roche e411 immunoassay analyzer (Roche Diagnostics Corporation, Indianapolis, IN) (borderline or reactive = 1, nonreactive = 0) was used to assess CMV seropositivity (Crimmins et al., 2017).
Controls
The current study is focused on the roles of chronic stress, CMV, and immune aging in mortality and multimorbidity independent of demographic factors and health behaviors. Therefore, I controlled for additional variables that may be confounds of the hypothesized associations. Specifically, I controlled for chronological age, race/ethnicity (Black, not Hispanic, Hispanic, other race, not Hispanic, and White, not Hispanic as the reference group), gender (Female = 1), educational attainment (less than 12 years, 12 years, 13–15 years, and 16 or more years as the reference group), self-reported smoking (current smoker, past smoker, or never smoked as the reference group), and body mass index (BMI; underweight, overweight, obese (obese I), morbidly obese (obese II), and normal weight as the reference group). To ease computation, age was divided by 10. These variables were regressed on health outcomes, immune aging variables, chronic stress, and CMV seropositivity.
Plan of Analysis
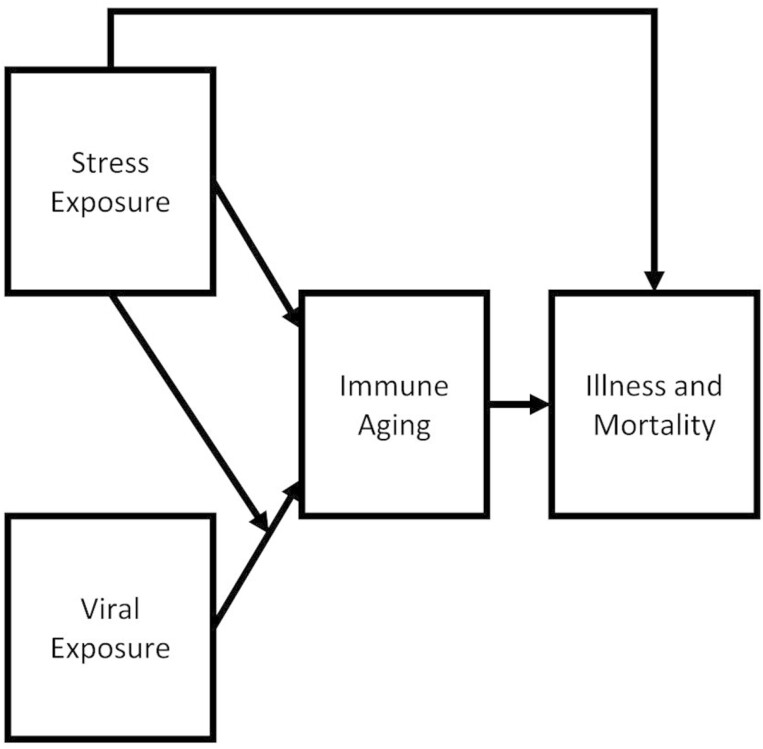
To assess the theoretical model shown in Figure 1, I estimated two structural equation models (SEMs) (1) regressing either mortality or multimorbidity on both CD4+ naïve and CD8+ naïve T cell percentages, chronic stress, CMV seropositivity, an interaction term multiplying chronic stress by CMV seropositivity and (2) regressing CD4+ naïve and CD8+ naïve T cell percentages on chronic stress, CMV seropositivity, and the interaction term. Models were estimated in Mplus 8.6 (Muthén & Muthén, 1998). All models here are fully recursive. Control variables were regressed on health outcomes, immune aging variables, chronic stress, CMV seropositivity, and the interaction term. I also estimated alternative models with different specifications that produced highly similar results, including models without health behavior control variables and without the interaction term, suggesting these results are relatively robust to alternative model specifications (see Sensitivity and Supplemental Analyses later and Supplementary Material).
Figure 1.
Theoretical model.
Because indirect effects are likely nonnormally distributed, tests of mediation and moderated mediation were performed using 95% percent confidence intervals using a bias-corrected bootstrap procedure with 1,000 draws. Moderated mediation here refers to an interaction involving a mediation effect. SEM is an ideal technique for the current study, as it allows for simultaneous estimation of all direct, mediating, and moderating effects. SEM also allows for formal testing of mediation using the nonparametric bootstrapping procedure as described previously. SEM has been used extensively in past research focused on mediation and moderation effects (Byrne, 2012; Cheung & Lau, 2017; Duncan, 1966).
Of the 9,934 participants in the HRS VBS, 718 participants were in a nursing home or were cohort ineligible (e.g., were too young) in 2016 and 221 participants were missing one or more independent (i.e., variables that are not a dependent variable in a regression in the model) variables. Weighted least squares mean and variance adjusted estimation with a probit-link function and delta parameterization was used. Thus, the final sample size is 8,995. Survey weights designed to make the sample population representative and provided by HRS were used. CMV seropositivity and mortality were treated as dichotomous variables using the “CATEGORICAL ARE” command in Mplus. To make results comparable across models, standardized results are shown. Models were fully recursive, so fit statistics are all uninformatively perfect.
Results
Descriptive Statistics
Descriptive statistics are shown in Table 1, Panel A. The weighted sample had a mean age of 68.60. A total of 78.36% of the sample was White, not Hispanic; 9.93% was Black, not Hispanic; 8.61% was Hispanic; and 3.10% was a member of another race, not Hispanic. A total of 46.11% of the sample was male participants and 53.88% of the sample was female participants. A total of 14.23% of the sample had fewer than 12 years of education, 29.25% had 12 years of education, 25.22% had 13–15 years of education, and 31.29% had 16 or more years of education. A total of 1.49% of the sample was underweight according to BMI, 25.21% was normal weight, 36.98% was overweight, 22.50% was obese I, and 13.83% was obese II. A total of 44.69% of respondents reported never smoking, 11.10% were current smokers, and 44.21% were former smokers.
Table 1.
Descriptive statistics and correlations among study variables
| Panel A: descriptive statistics | |||||||
|---|---|---|---|---|---|---|---|
| Mean/proportion | SD | Range | |||||
| Mortality | 0.10 | ||||||
| Multimorbidity | 2.09 | 1.38 | 0–7 | ||||
| % CD4+ naïve | 0.45 | 0.18 | 0–0.95 | ||||
| % CD8+ naïve | 0.23 | 0.16 | 0–0.90 | ||||
| Chronic stress | 0.00 | 3.82 | −4.64–18.36 | ||||
| CMV seropositivity | 0.63 | ||||||
| Age | 68.60 | 9.19 | 56–107 | ||||
| Race/ethnicity | |||||||
| White, not Hispanic | 0.78 | ||||||
| Black, not Hispanic | 0.10 | ||||||
| Hispanic | 0.09 | ||||||
| Other race, not Hispanic | 0.03 | ||||||
| Gender | |||||||
| Male | 0.46 | ||||||
| Female | 0.54 | ||||||
| Education | |||||||
| 0–11 years | 0.14 | ||||||
| 12 years | 0.29 | ||||||
| 13–15 years | 0.25 | ||||||
| 16+ years | 0.31 | ||||||
| BMI | |||||||
| Underweight | 0.01 | ||||||
| Normal weight | 0.25 | ||||||
| Overweight | 0.37 | ||||||
| Obese I | 0.22 | ||||||
| Obese II | 0.14 | ||||||
| Smoker status | |||||||
| Never smoked | 0.45 | ||||||
| Current smoker | 0.11 | ||||||
| Past smoker | 0.44 | ||||||
| Panel B: correlations among study variables | |||||||
| I | Mortality | 1.00 | |||||
| II | Multimorbidity | 0.22*** | 1.00 | ||||
| III | % CD4+ naïve | −0.07*** | −0.17*** | 1.00 | |||
| IV | % CD8+ naïve | −0.13*** | −0.15*** | 0.44*** | 1.00 | ||
| V | Chronic stress | 0.01 | 0.21*** | −0.06*** | 0.04*** | 1.00 | |
| VI | CMV seropositivity | 0.08*** | 0.10*** | −0.22*** | −0.32*** | 0.07*** | 1.00 |
| I | II | III | IV | V | VI | ||
Note: ***p < .001. BMI = body mass index; CMV = cytomegalovirus; SD = standard deviation.
A total of 9.54% of respondents died between 2016 and 2020. Participants had an average of 2.09 chronic illnesses. For an average respondent, 44.51% of their CD4+ cells were naïve cells and 22.70% of their CD8+ cells were naïve cells. Chronic stress was mean centered. Before this transformation, the average participant had a chronic stress score of 12.64. A total of 63.39% respondents were CMV-positive. Pairwise Pearson correlations among variables are shown in Table 1, Panel B. Except for the association between mortality and chronic stress, all correlations are highly significant (p < .001). These associations are explored in the multivariate analysis subsequently.
Mortality
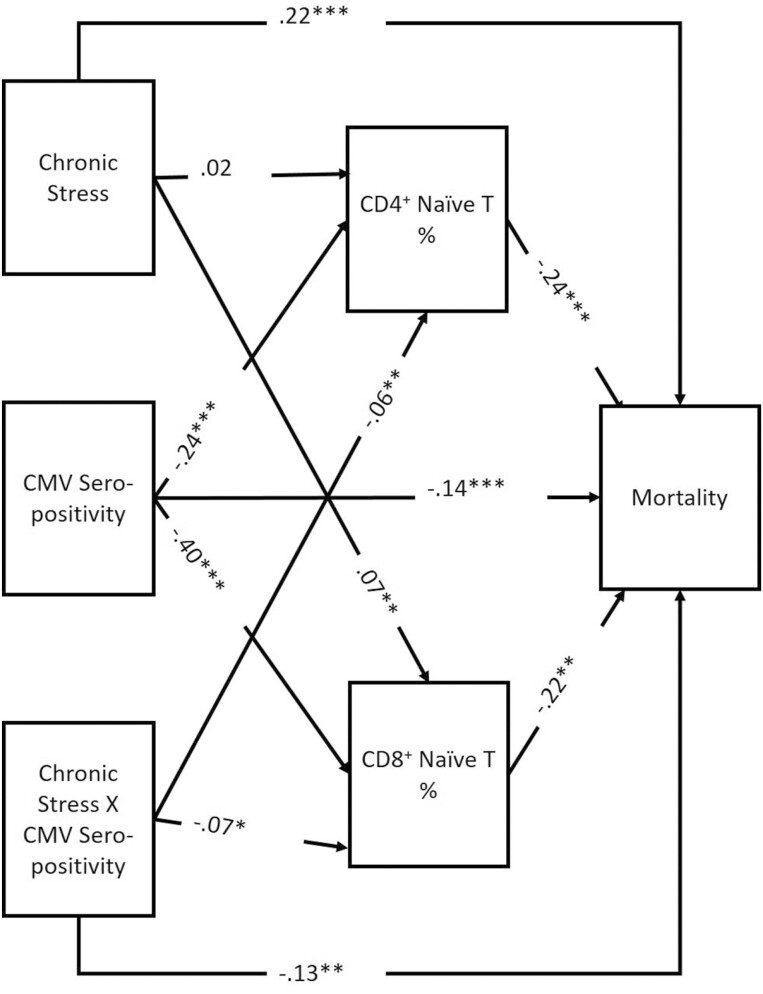
Results for the SEM with mortality as the outcome are shown in Figure 2. As noted above, this model is fully recursive, and therefore model fit statistics are uninformatively perfect. In this model, mortality risk was associated with both the percentage of CD4+ (β = −0.235, 95% CI: −0.263, −0.222) and CD8+ naïve T cells (β = −0.224, 95% CI: − 0.263, −0.195). Mortality was positively associated with chronic stress (β = 0.219, 95% CI: 0.119, 0.327) and was negatively associated with CMV seropositivity (β = −0.134, 95% CI: − 0.242, −0.039) and the interaction term between chronic stress and CMV seropositivity (β = −0.138, 95% CI: − 0.204, −0.067).
Figure 2.
Structural equation model (SEM) mortality results. χ2 = 00(d.f.), p = 0; CFI = 1; RMSEA = 0; N = 8,995; *p < .05, **p < .01, ***p < .001. CFI = comparative fit index; CMV = cytomegalovirus; RMSEA = root mean square error of approximation.
As expected, CMV seropositivity was significantly negatively associated with both the percentage of CD4+ (β = −0.241, 95% CI: − 0.276, −0.206) and CD8+ (β = −0.400, 95% CI: − 0.422, −0.347) naïve T cells. The interaction term between chronic stress and CMV seropositivity was negatively associated with the percentages of CD4+ (β = −0.057, 95% CI: − 0.115, −0.006) and CD8+ (β = −0.071, 95% CI: − 0.125, −0.015) naïve T cells. Experiencing greater chronic stress was associated with a greater percentage of CD8+ naïve T cells (β = 0.074, 95% CI: 0.017, 0.124); however, in an alternative model without the interaction term (see Supplementary Material), chronic stress is not significantly associated with naïve cell percentage, suggesting that the association between chronic stress and immune aging may be due to its nonlinear interaction with CMV. These results are further explicated in the moderated moderation section later.
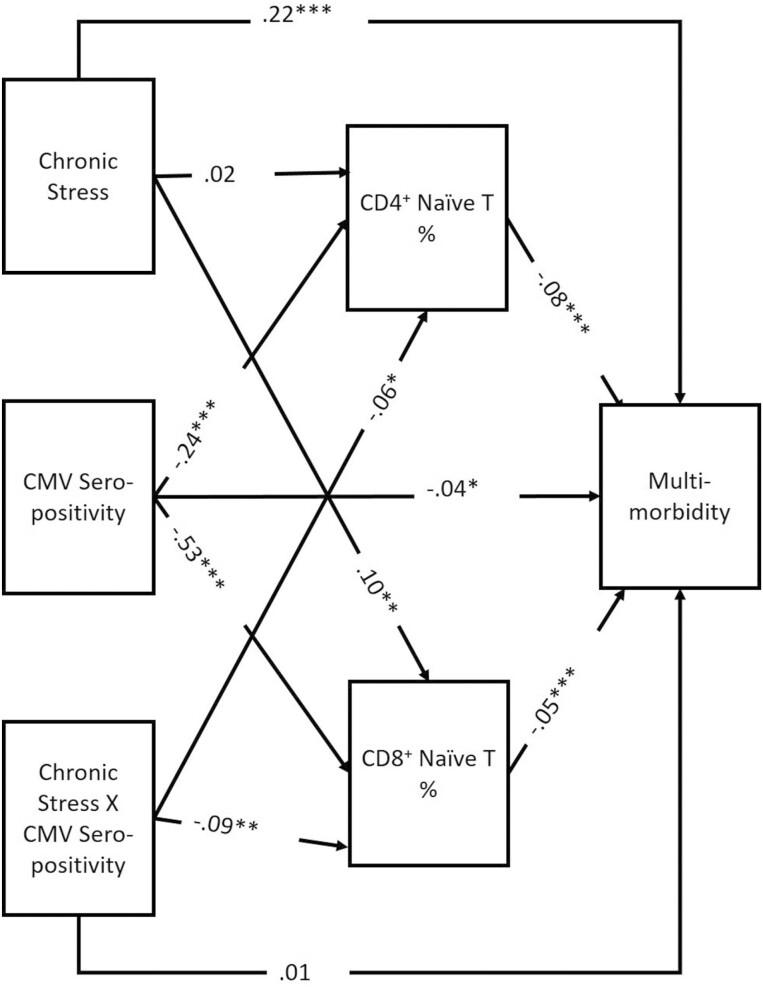
Multimorbidity
Results for the SEM with multimorbidity as the outcome are shown in Figure 3. In this model, the number of chronic illnesses was negatively associated with both the percentage of CD4+ (β = −0.081, 95% CI: − 0.090, −0.056) and CD8+ (β = −0.048, 95% CI: − 0.059, −0.039) naïve T cells. Multimorbidity was positively associated with chronic stress (β = 0.219, 95% CI: 0.174, 0.263) and was negatively associated with CMV seropositivity (β = −0.044, 95% CI: − 0.090, −0.003). As in the previous model, CMV seropositivity and the interaction term between chronic stress were negatively associated with the percentages of CD4+ and naïve T cells, and chronic stress was positively associated with the percentage of CD8+ naïve T cells; again, however, in an alternative model without the interaction term (see Supplementary Material), chronic stress is not significantly associated with naïve cell percentage.
Figure 3.
SEM multimorbidity results. χ2 = 00(d.f.), p = 0; CFI = 1; RMSEA = 0; N = 8,995; standardized results shown; *p < .05, **p < .01, ***p < .001. CMV = cytomegalovirus.
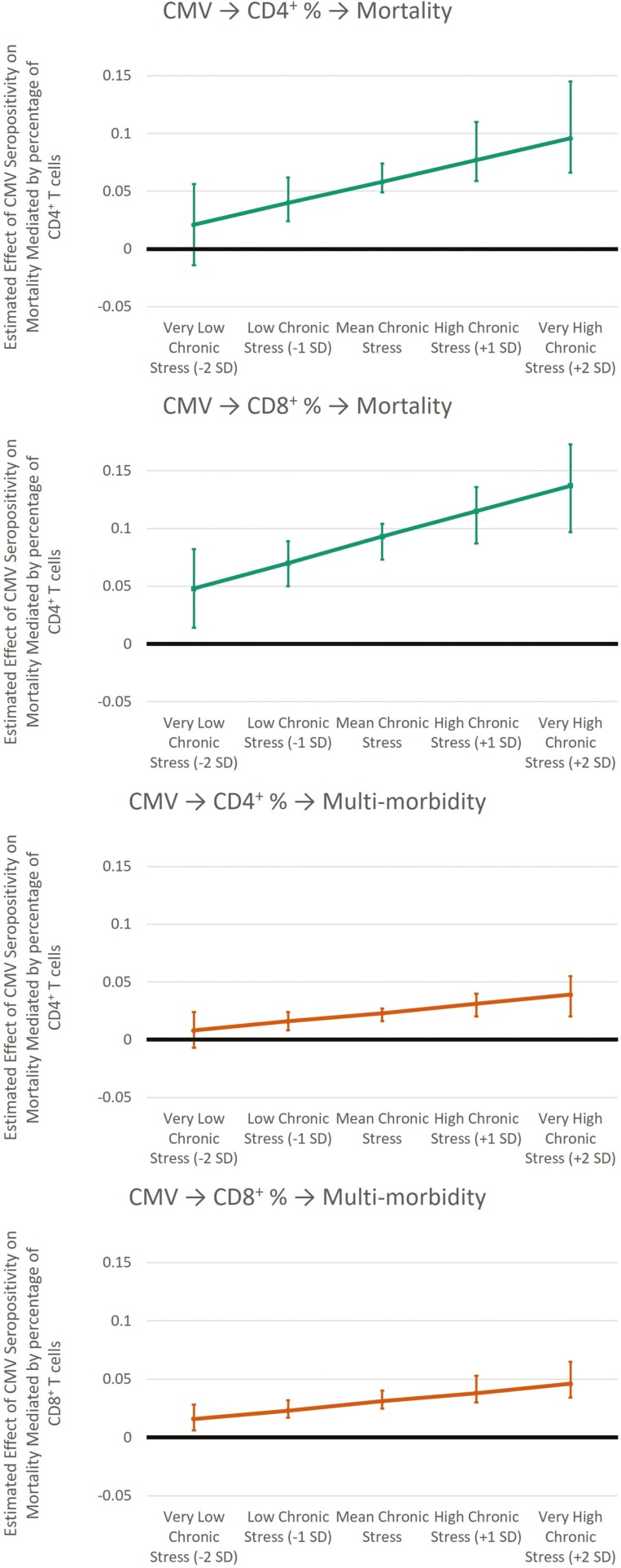
Moderated Mediation
As noted above, the interaction term between chronic stress and CMV seropositivity was significantly associated with both CD4+ and CD8+ naïve T cells in both models. This suggests that social stress amplifies the immune aging effect of CMV. This argument is further supported by moderated mediation analysis (Figure 4). For the mortality model, the indirect effects of CMV seropositivity on mortality as mediated by both CD4+ and CD8+ naïve T cells are amplified by chronic stress. That is, the indirect effect via CD4+ naïve cell percentage was stronger for participants who reported more chronic stress (at −1 standard deviation: b = 0.040, 95% CI: 0.024, 0.062; at +1 standard deviation: b = 0.077, 95% CI: 0.059, 0.110), and the same is true for the indirect path via CD8+ naïve cell percentage (at −1 standard deviation: b = 0.070, 95% CI: 0.050, 0.089; at +1 standard deviation: b = 0.115, 95% CI: 0.087, 0.136). There was a similar pattern of results for multimorbidity. The indirect effect via CD4+ naïve cell percentage was stronger for participants who reported more chronic stress (at −1 standard deviation: b = 0.016, 95% CI: 0.008, 0.024; at +1 standard deviation: b = 0.031, 95% CI: 0.020, 0.040), and the same is true for the indirect path via CD8+ naïve cell percentage (at −1 standard deviation: b = 0.023, 95% CI: 0.017, 0.032; at +1 standard deviation: b = 0.038, 95% CI: 0.030, 0.053). At very low levels of chronic stress (−2 standard deviations), the indirect paths including CD4+ naïve cell percentage are no longer significant for either mortality risk or multimorbidity. These results suggest that CMV infections may be more active for people who experience more chronic stress in later life, potentially leading to accelerated immune aging, chronic disease morbidity, and mortality.
Figure 4.
Estimated effects from moderated mediation analysis. Nonstandardized results shown. 95% confidence intervals from bias-corrected bootstrap procedure using 1,000 draws. CMV = cytomegalovirus; SD = standard deviation.
Sensitivity and Supplemental Analyses
Using a count of illness is a common method for assessing multimorbidity; however, past research has also utilized indices of morbidities weighted by severity and/or mortality risk. According to a recent systematic review, different multimorbidity methods tend to have similar predictive validity (Huntley et al., 2012). As a sensitivity analysis, I estimated the multimorbidity model using a modified version of the Seattle index of comorbidities (SIC), a measure that has been validated in past research and can be calculated in large observational studies using self-reported disease status, like HRS (Fan et al., 2002). I could not perfectly reproduce the SIC, as HRS does not have information about pneumonia, and congestive heart failure and myocardial infarction were not differentiated. Therefore, I used the following formula:
| (1) |
Results were very similar to the main analysis and would not lead to different substantive conclusions.
Mortality information was collected throughout 2020, including the beginning of the COVID-19 pandemic in the United States. The same processes investigated in this study leading to all-cause mortality should similarly lead to COVID-specific mortality. However, to ensure that COVID-specific mortality is not inflating results, I also estimated the mortality model excluding all participants that died in 2020. Results were very similar with an identical pattern of significance, suggesting that results are not biased by the COVID-19 pandemic.
Discussion
Past research suggests that stress accelerates immune aging and may reactivate latent viral infections, leading to chronic disease morbidity and mortality (Klopack, Crimmins, et al., 2022). Therefore, researchers have argued that immune aging and exposure to latent viruses (including CMV) may be a pathway in the association between stress and health. The link between chronic stress exposure and health is well established; however, the biological pathways underlying the stress process are poorly understood. Studies like this one can help uncover the black box underlying the stress process. To that end, this study focused on immune aging (as characterized by percentages of naïve T lymphocytes) as a window into the biological processes underlying stress and health pathways.
Results suggest that immune aging is a potentially important biological pathway underlying the stress process. That is, I found that having higher percentages of CD4+ and CD8+ naïve T cells (indicative of less immune aging) was associated with a lower risk of mortality and lower chronic disease morbidity. Consistent with past research, I found that CMV seropositivity was associated with substantially lower naïve T cell percentages (Noppert et al., 2023). This effect was amplified by chronic stress, such that at higher levels of chronic stress, CD4+ and CD8+ naïve cell percentages mediated a larger portion of the health risk associated with CMV seropositivity. To my knowledge, this is the first study to report this moderated mediation effect and the first study to report an interaction between stress and CMV seropositivity in a population-representative sample.
There is evidence in past research that chronic stress is particularly important in the development of chronic illness and mortality (Crielaard et al., 2021). I found the interaction between chronic stress and CMV was associated with a lower proportion of naïve T cells, and ultimately with chronic illness multimorbidity and mortality. This study may suggest viral reactivation is one pathway explaining the importance of chronic stress. That is, for people who are CMV-positive, the reactivation of CMV by chronic stress may increase the relative importance of chronic stress in driving health problems. Future research is needed to confirm this finding and to assess whether other stressors similarly affect CMV and immune aging. Additionally, these findings may help explain differential vulnerability to stress among structurally marginalized groups (Thoits, 2010). Because individuals that are minoritized are structurally more likely to be exposed to conditions that put them at greater risk of viral exposure, they are more likely to have a greater viral burden compared with nonminoritized individuals (Meier et al., 2016; Noppert et al., 2021). The results here suggest that these individuals are then more substantially affected by chronic stress because that stress reactivates latent viral infections. Future research testing this hypothesis could help clarify why socially marginalized groups tend to be more affected by stress.
Past research has found that social structural factors, like socioeconomic status, and social experiences can have persistent effects on health at later stages in the life course (Ferraro et al., 2016; Morton & Ferraro, 2020). The findings of the current study reflect a body of emerging research suggest that accelerated immune aging may help explain how these factors affect health years later (Klopack, Thyagarajan, et al., 2022; Noppert et al., 2021, 2023). SES is strongly associated with exposure to viral infections; thus, SES in early life and midlife increases the risk of viral infections that may drive immune aging and ultimately health problems in later life. More research with data on life course exposure to viruses like CMV is needed.
Similarly, chronic stress experienced in earlier life stages may reactivate latent viruses like CMV, accelerating immune aging. A more aged immune system is less able to fight infections, clear cancerous cells, and effectively regulate inflammation (Aiello et al., 2019). Thus, immune aging may help explain past findings that early life stress can amplify the effects of later life stress on health (Simons et al., 2019). Consistent with cumulative disadvantage theory and cumulative inequality theory—that is, disadvantages at one point in life can drive and potentially amplify more disadvantages in later life (Ferraro & Kelley-Moore, 2003; Ferraro et al., 2016)—social marginalized people are at greater risk of CMV exposures, leading to a more aged immune system that is less able to fight new infections and is less able to regulate inflammatory processes, leading to chronic illnesses. These chronic illnesses can generate stress, further damaging immune functioning and reactivating CMV. Thus, CMV and immune aging may be a useful window to understand how structured social inequalities lead to environmental exposures that may amplify the health risk associated with stress. Again, more research with life course data on stress and immune functioning is needed.
Addressing the link between social inequalities and health hazard exposures (e.g., viral exposures, social stress, and air pollution) could help reduce inequalities in health and illness. I found an interaction between viral exposure and stress on health outcomes. In the specific case of CMV studied here, the development and widespread distribution of effective vaccines could help weaken the link between stress and morbidity and mortality. No vaccine for CMV is currently available, but a number are in development, including some in Phase 2 clinical trials (Inoue et al., 2018). Widespread immunization against CMV could help reduce the health burden associated with chronic stress. Though the most effective way to reduce health inequalities is likely to address the underlying fundamental social inequalities, this research also highlights potential areas for individual intervention. For adults who experience high levels of stress, interventions focused on reducing chronic stress or building resilience and coping tools could be beneficial. Additionally, effective CMV-specific antiviral treatments could help weaken the link between stress and health.
Limitations
CMV seroprevalence and flow cytometry were only assessed at one time. Longitudinal assessments are needed to establish the temporal ordering of CMV infection and age-related cell type percentages and ratios. For count outcomes, I used linear regression with bootstrap standard errors. Multimorbidity cannot be declared as a count and CMV as dichotomous in the same model. However, bootstrap standard errors should be robust to nonnormality. Additionally, I estimated an alternative model using a continuous scale of multimorbidity (SIC) that had very similar results. This study is limited to U.S. adults 56 years and older. Studies in other age groups and other countries are needed. This study only investigated CMV seropositivity. Other chronic viral infections and other early life adversity associated health-risk exposures may also play a role in life course stress interactions. Because past research suggests they may be most implicated in immune aging, I focus on naïve T cells; however, other immune cell types may be important for these processes.
Conclusion
Despite these limitations, this study expands the literature on social determinants in health inequalities. This study illuminates the biological mechanisms underlying emerging research on the social pathways by which stress affects health and illness. This research shows how biological and social processes can be integrated to explicate social inequalities more clearly and to better develop interventions to address these inequalities. Focusing on how social inequalities get “under the skin,” may help identify for whom and under what conditions social forces affect biological functioning and health, and may help identify large-scale interventions to reduce health inequalities (e.g., CMV vaccination). The careful integration of biological and social data may be helpful for researchers interested in reducing social inequalities.
Supplementary Material
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers T32AG000037 and P30AG017265. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Health and Retirement Study (HRS) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan.
Conflict of Interest
None.
References
- Aiello, A. E., Dowd, J. B., Jayabalasingham, B., Feinstein, L., Uddin, M., Simanek, A. M., Cheng, C. K., Galea, S., Wildman, D. E., Koenen, K., & Pawelec, G. (2016). PTSD is associated with an increase in aged T cell phenotypes in adults living in Detroit. Psychoneuroendocrinology, 67, 133–141. doi: 10.1016/j.psyneuen.2016.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello, A. E., Feinstein, L., Dowd, J. B., Pawelec, G., Derhovanessian, E., Galea, S., Uddin, M., Wildman, D. E., & Simanek, A. M. (2016). Income and markers of immunological cellular aging. Psychosomatic Medicine, 78(6), 657–666. doi: 10.1097/PSY.0000000000000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello, A., Farzaneh, F., Candore, G., Caruso, C., Davinelli, S., Gambino, C. M., Ligotti, M. E., Zareian, N., & Accardi, G. (2019). Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Frontiers in Immunology, 10, 1–19. doi: 10.3389/fimmu.2019.02247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, C. R., Wolf, J., Brunner, S., Herndler-Brandstetter, D., & Grubeck-Loebenstein, B. (2011). Gain and loss of T cell subsets in old age—Age-related reshaping of the T cell repertoire. Journal of Clinical Immunology, 31(2), 137–146. doi: 10.1007/s10875-010-9499-x [DOI] [PubMed] [Google Scholar]
- Aronoff, J. E., Quinn, E. B., Forde, A. T., Glover, L. M., Reiner, A., McDade, T. W., & Sims, M. (2022). Associations between perceived discrimination and immune cell composition in the Jackson Heart Study. Brain, Behavior, and Immunity, 103, 28–36. doi: 10.1016/j.bbi.2022.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley, I., Pera, A., Morrow, G., Davies, K. A., & Kern, F. (2017). Expansions of cytotoxic CD4+CD28− T cells drive excess cardiovascular mortality in rheumatoid arthritis and other chronic inflammatory conditions and are triggered by CMV infection. Frontiers in Immunology, 8, 1–10. doi: 10.3389/fimmu.2017.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, B. M. (2012). Structural equation modeling with Mplus: Basic concepts, applications, and programming. Routledge; cat00002a. http://proxy-remote.galib.uga.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=cat00002a&AN=gua3908413&site=eds-live [Google Scholar]
- Cannon, M., Schmid, D., & Hyde, T. (2010). Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Reviews in Medical Virology, 20(4), 202–213. doi: 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- Chen, S., Pawelec, G., Trompet, S., Goldeck, D., Mortensen, L. H., Slagboom, P. E., Christensen, K., Gussekloo, J., Kearney, P., Buckley, B. M., Ford, I., Jukema, J. W., Westendorp, R. G. J., & Maier, A. B. (2021). Associations of cytomegalovirus infection with all-cause and cardiovascular mortality in multiple observational cohort studies of older adults. Journal of Infectious Diseases, 223(2), 238–246. doi: 10.1093/infdis/jiaa480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, G. W., & Lau, R. S. (2017). Accuracy of parameter estimates and confidence intervals in moderated mediation models: A comparison of regression and latent moderated structural equations. Organizational Research Methods, 20(4), Article 4. EDSWSS. doi: 10.1177/1094428115595869 [DOI] [Google Scholar]
- Cole, S. W. (2019). The conserved transcriptional response to adversity. Current Opinion in Behavioral Sciences, 28, 31–37. doi: 10.1016/j.cobeha.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colugnati, F. A., Staras, S. A., Dollard, S. C., & Cannon, M. J. (2007). Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infectious Diseases, 7(1), 71. doi: 10.1186/1471-2334-7-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crielaard, L., Nicolaou, M., Sawyer, A., Quax, R., & Stronks, K. (2021). Understanding the impact of exposure to adverse socioeconomic conditions on chronic stress from a complexity science perspective. BMC Medicine, 19(1), 242. doi: 10.1186/s12916-021-02106-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins, E. M., Faul, J. D., Thyagarajan, B., & Weir, D. R. (2017). Venous blood collection and assay protocol in the 2016 Health and Retirement Study 2016 Venous Blood Study (VBS).https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/HRS2016VBSDD.pdf
- de Felice, C., Toti, P., Musarò, M., Peruzzi, L., Paffetti, P., Pasqui, L., Magaldi, R., Bagnoli, F., Rinaldi, M., Rinaldi, G., Grilli, G., Tonni, G., & Latini, G. (2008). Early activation of the hypothalamic–pituitary–adrenal axis in very-low-birth-weight infants with small thymus at birth. Journal of Maternal–Fetal & Neonatal Medicine, 21(4), 251–254. doi: 10.1080/14767050801927871 [DOI] [PubMed] [Google Scholar]
- Dowd, J. B., Aiello, A. E., & Alley, D. E. (2009). Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiology and Infection, 137(1), 58–65. doi: 10.1017/S0950268808000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggal, N. A., Pollock, R. D., Lazarus, N. R., Harridge, S., & Lord, J. M. (2018). Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell, 17(2), e12750. doi: 10.1111/acel.12750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, O. D. (1966). Path analysis: Sociological examples. American Journal of Sociology, 72(1), 1–16. doi: 10.1086/224256 [DOI] [Google Scholar]
- Elwenspoek, M. M. C., Sias, K., Hengesch, X., Schaan, V. K., Leenen, F. A. D., Adams, P., Mériaux, S. B., Schmitz, S., Bonnemberger, F., Ewen, A., Schächinger, H., Vögele, C., Muller, C. P., & Turner, J. D. (2017). T cell immunosenescence after early life adversity: Association with cytomegalovirus infection. Frontiers in Immunology, 8, 1–12. doi: 10.3389/fimmu.2017.01263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, M. K. (2020). Covid’s color line—Infectious disease, inequity, and racial justice. New England Journal of Medicine, 383(5), 408–410. doi: 10.1056/nejmp2019445 [DOI] [PubMed] [Google Scholar]
- Fan, V. S., Au, D., Heagerty, P., Deyo, R. A., McDonell, M. B., & Fihn, S. D. (2002). Validation of case-mix measures derived from self-reports of diagnoses and health. Journal of Clinical Epidemiology, 55(4), 371–380. doi: 10.1016/S0895-4356(01)00493-0 [DOI] [PubMed] [Google Scholar]
- Ferraro, K. F., & Kelley-Moore, J. A. (2003). Cumulative disadvantage and health: Long-term consequences of obesity? American Sociological Review, 68(5), Article 5. doi: 10.2307/1519759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro, K. F., Schafer, M. H., & Wilkinson, L. R. (2016). Childhood disadvantage and health problems in middle and later life: Early imprints on physical health? American Sociological Review, 81(1), Article 1. doi: 10.1177/0003122415619617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, B. N., Teague, T. K., Bayouth, M., Yolken, R. H., Bodurka, J., Irwin, M. R., Paulus, M. P., & Savitz, J. (2020). Diagnosis-independent loss of T-cell costimulatory molecules in individuals with cytomegalovirus infection. Brain, Behavior, and Immunity, 87, 795–803. doi: 10.1016/j.bbi.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop, T., Larbi, A., Dupuis, G., Le Page, A., Frost, E. H., Cohen, A. A., Witkowski, J. M., & Franceschi, C. (2018). Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Frontiers in Immunology, 8, 1960. doi: 10.3389/fimmu.2017.01960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser, R., & Kiecolt-Glaser, J. K. (1997). Chronic stress modulates the virus-specific immune response to latent herpes simplex virus Type 1. Annals of Behavioral Medicine, 19(2), 78–82. doi: 10.1007/bf02883323 [DOI] [PubMed] [Google Scholar]
- Hayward, S. E., Dowd, J. B., Fletcher, H., Nellums, L. B., Wurie, F., & Boccia, D. (2020). A systematic review of the impact of psychosocial factors on immunity: Implications for enhancing BCG response against tuberculosis. SSM—Population Health, 10, 100522. doi: 10.1016/j.ssmph.2019.100522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health and Retirement Study. (2021). Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number U01AG009740), Ann Arbor, MI. https://hrs.isr.umich.edu/
- Hoes, J., Boef, A. G. C., Knol, M. J., de Melker, H. E., Mollema, L., van der Klis, F. R. M., Rots, N. Y., & van Baarle, D. (2018). Socioeconomic status is associated with antibody levels against vaccine preventable diseases in the Netherlands. Frontiers in Public Health, 6, 209. doi: 10.3389/fpubh.2018.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez, P. J. (2008). Neglected infections of poverty in the United States of America. PLoS Neglected Tropical Diseases, 2(6), e256. doi: 10.1371/journal.pntd.0000256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff, W. X., Kwon, J. H., Henriquez, M., Fetcko, K., & Dey, M. (2019). The evolving role of CD8+CD28− immunosenescent T cells in cancer immunology. International Journal of Molecular Sciences, 20(11), 2810. doi: 10.3390/ijms20112810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley, A. L., Johnson, R., Purdy, S., Valderas, J. M., & Salisbury, C. (2012). Measures of multimorbidity and morbidity burden for use in primary care and community settings: A systematic review and guide. The Annals of Family Medicine, 10(2), 134–141. doi: 10.1370/afm.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, N., Abe, M., Kobayashi, R., & Yamada, S. (2018). Vaccine development for cytomegalovirus. In Kawaguchi Y., Mori Y., & Kimura H. (Eds.), Human herpesviruses (pp. 271–296). Springer. doi: 10.1007/978-981-10-7230-7_13 [DOI] [PubMed] [Google Scholar]
- Kananen, L., Nevalainen, T., Jylhävä, J., Marttila, S., Hervonen, A., Jylhä, M., & Hurme, M. (2015). Cytomegalovirus infection accelerates epigenetic aging. Experimental Gerontology, 72, 227–229. doi: 10.1016/j.exger.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Klopack, E. T., Crimmins, E. M., Cole, S. W., Seeman, T. E., & Carroll, J. E. (2022). Social stressors associated with age-related T lymphocyte percentages in older US adults: Evidence from the US Health and Retirement Study. Proceedings of the National Academy of Sciences, 119(25), e2202780119. doi: 10.1073/pnas.2202780119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopack, E. T., Thyagarajan, B., Faul, J. D., Meier, H. C. S., Ramasubramanian, R., Kim, J. K., & Crimmins, E. M. (2022). Socioeconomic status and immune aging in older US adults in the Health and Retirement Study. Biodemography and Social Biology, 0(0), 1–16. doi: 10.1080/19485565.2022.2149465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz, P. M., House, J. S., Mero, R. P., & Williams, D. R. (2005). Stress, life events, and socioeconomic disparities in health: Results from the Americans’ Changing Lives Study. Journal of Health and Social Behavior, 46(3), 274–288. doi: 10.1177/002214650504600305 [DOI] [PubMed] [Google Scholar]
- Maecker, H. T., McCoy, J. P., & Nussenblatt, R. (2012). Standardizing immunophenotyping for the Human Immunology Project. Nature Reviews Immunology, 12(3), 191–200. doi: 10.1038/nri3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase, E. (2022). Monkeypox: What do we know about the outbreaks in Europe and North America? BMJ, 377, o1274. doi: 10.1136/bmj.o1274 [DOI] [PubMed] [Google Scholar]
- McEwen, B. S., & Seeman, T. (1999). Protective and damaging effects of mediators of stress: Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences, 896(1), 30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x [DOI] [PubMed] [Google Scholar]
- McEwen, C. A., & McEwen, B. S. (2017). Social structure, adversity, toxic stress, and intergenerational poverty: An early childhood model. Annual Review of Sociology, 43(1), 445–472. doi: 10.1146/annurev-soc-060116-053252 [DOI] [Google Scholar]
- Meier, H. C. S., Haan, M. N., Mendes de Leon, C. F., Simanek, A. M., Dowd, J. B., & Aiello, A. E. (2016). Early life socioeconomic position and immune response to persistent infections among elderly Latinos. Social Science & Medicine, 166, 77–85. doi: 10.1016/j.socscimed.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett, G. A., Jones, A. T., Benkeser, D., Baral, S., Mercer, L., Beyrer, C., Honermann, B., Lankiewicz, E., Mena, L., Crowley, J. S., Sherwood, J., & Sullivan, P. S. (2020). Assessing differential impacts of COVID-19 on black communities. Annals of Epidemiology, 47, 37–44. doi: 10.1016/j.annepidem.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton, P. M., & Ferraro, K. F. (2020). Early social origins of biological risks for men and women in later life. Journal of Health and Social Behavior, 61(4), 503–522. doi: 10.1177/0022146520966364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, L. K., & Muthén, B. O. (1998). Mplus user’s guide (7th ed.). Muthén & Muthén. [Google Scholar]
- Needham, B. L., Adler, N., Gregorich, S., Rehkopf, D., Lin, J., Blackburn, E. H., & Epel, E. S. (2013). Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Social Science & Medicine, 85, 1–8. doi: 10.1016/j.socscimed.2013.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppert, G. A., Stebbins, R. C., Beam Dowd, J., & Aiello, A. E. (2023). Socioeconomic and race/ethnic differences in immunosenescence: Evidence from the Health and Retirement Study. Brain, Behavior, and Immunity, 107, 361–368. doi: 10.1016/j.bbi.2022.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppert, G. A., Stebbins, R. C., Dowd, J. B., Hummer, R. A., & Aiello, A. E. (2021). Life course socioeconomic disadvantage and the aging immune system: Findings from the Health and Retirement Study. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 76(6), 1195–1205. doi: 10.1093/geronb/gbaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangrazzi, L., & Weinberger, B. (2020). T cells, aging and senescence. Experimental Gerontology, 134, 110887. doi: 10.1016/j.exger.2020.110887 [DOI] [PubMed] [Google Scholar]
- Pawelec, G., McElhaney, J. E., Aiello, A. E., & Derhovanessian, E. (2012). The impact of CMV infection on survival in older humans. Current Opinion in Immunology, 24(4), 507–511. doi: 10.1016/j.coi.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Pearlin, L. I. (2010). The life course and the stress process: Some conceptual comparisons. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65B(2), Article 2. doi: 10.1093/geronb/gbp106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather, A. A., Epel, E. S., Portela Parra, E., Coccia, M., Puterman, E., Aiello, A. E., & Dhabhar, F. S. (2018). Associations between chronic caregiving stress and T cell markers implicated in immunosenescence. Brain, Behavior, and Immunity, 73, 546–549. doi: 10.1016/j.bbi.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubramanian, R., Meier, H. C. S., Vivek, S., Klopack, E., Crimmins, E. M., Faul, J., Nikolich-Žugich, J., & Thyagarajan, B. (2022). Evaluation of T-cell aging-related immune phenotypes in the context of biological aging and multimorbidity in the Health and Retirement Study. Immunity & Ageing, 19(1), 33. doi: 10.1186/s12979-022-00290-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, R. G., Presnell, S. R., Al-Attar, A., Lutz, C. T., & Segerstrom, S. C. (2019). Perceived stress, cytomegalovirus titers, and late-differentiated T and NK cells: Between-, within-person associations in a longitudinal study of older adults. Brain, Behavior, and Immunity, 80, 266–274. doi: 10.1016/j.bbi.2019.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom, S. C., & Miller, G. E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630. doi: 10.1037/0033-2909.130.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serido, J., Almeida, D. M., & Wethington, E. (2004). Chronic stressors and daily hassles: Unique and interactive relationships with psychological distress. Journal of Health and Social Behavior, 45(1), 17–33. doi: 10.1177/002214650404500102 [DOI] [PubMed] [Google Scholar]
- Simons, R. L., Lei, M. -K., Klopack, E., Beach, S. R. H., Gibbons, F. X., & Philibert, R. A. (2021). The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Social Science & Medicine, 282(1131), 69. doi: 10.1016/j.socscimed.2020.113169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, R. L., Woodring, D., Simons, L. G., Sutton, T. E., Lei, M. -K., Beach, S. R. H., Barr, A. B., & Gibbons, F. X. (2019). Youth adversities amplify the association between adult stressors and chronic inflammation in a domain specific manner: Nuancing the early life sensitivity model. Journal of Youth and Adolescence, 48, 1–16. doi: 10.1007/s10964-018-0977-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana, R., Tarazona, R., Aiello, A. E., Akbar, A. N., Appay, V., Beswick, M., Bosch, J. A., Campos, C., Cantisán, S., Cicin-Sain, L., Derhovanessian, E., Ferrando-Martínez, S., Frasca, D., Fulöp, T., Govind, S., Grubeck-Loebenstein, B., Hill, A., Hurme, M., Kern, F., & Pawelec, G. (2012). CMV and immunosenescence: From basics to clinics. Immunity & Ageing, 9(1), Article 1. doi: 10.1186/1742-4933-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro, O., Youm, Y., Shchukina, I., Ryu, S., Sidorov, S., Ravussin, A., Nguyen, K., Aladyeva, E., Predeus, A. N., Smith, S. R., Ravussin, E., Galban, C., Artyomov, M. N., & Dixit, V. D. (2022). Caloric restriction in humans reveals immunometabolic regulators of health span. Science, 375(6581), 671–677. doi: 10.1126/science.abg7292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras, S. A. S., Dollard, S. C., Radford, K. W., Flanders, W. D., Pass, R. F., & Cannon, M. J. (2006). Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clinical Infectious Diseases, 43(9), 1143–1151. doi: 10.1086/508173 [DOI] [PubMed] [Google Scholar]
- Thoits, P. A. (2010). Stress and health: Major findings and policy implications. Journal of Health and Social Behavior, 51, S41–S53. doi: 10.1177/0022146510383499 [DOI] [PubMed] [Google Scholar]
- Thyagarajan, B., Faul, J., Vivek, S., Kim, J. K., Nikolich-Žugich, J., Weir, D., & Crimmins, E. M. (2022). Age-related differences in T cell subsets in a nationally representative sample of people over age 55: Findings from the Health and Retirement Study. The Journals of Gerontology: Series A, 77(5), 927–933. doi: 10.1093/gerona/glab300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel, W. M., Matthews, K. A., Bromberger, J. T., & Sutton-Tyrrell, K. (2003). Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychology, 22(3), 300–309. doi: 10.1037/0278-6133.22.3.300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.