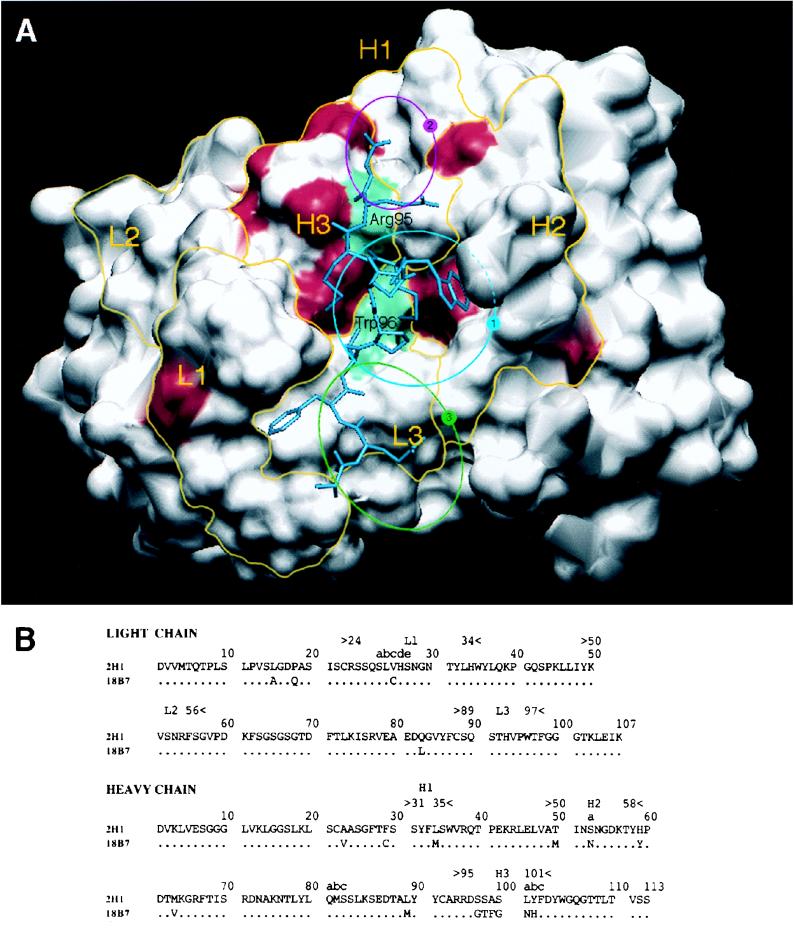
FIG. 1.
(A) Solvent-accessible surfaces of the MAb 2H1 binding site with the amino acid differences between MAbs 2H1 and 18B7 highlighted in red. L1, L2, and L3 refer to the three light chain CDRs. H1, H2, and H3 refer to the three heavy chain CDRs. Yellow lines denote the structural area associated with CDRs. The positions of arginine H95 in the VH CDR3 and tryptophan L96 in the VL CDR3 are shown in green. The position of the peptide mimotope PA1 is drawn in blue (50). The sequence of PA1 is GLQYTPSWMLVG. The blue circle labeled “1” denotes the location of the central hydrophobic pocket delimited by VH CDR1, CDR2, and CDR3 and VL CDR1 and -3. The magenta circle labeled “2” denotes the location of a small hydrophobic pocket containing arginine H95. The green circle labeled “3” denotes the location of a potential extension of the binding groove that is highly conserved in both MAbs 2H1 and 18B7. (B) Sequence comparison of heavy and light chain variable regions of MAbs 2H1 and 18B7 (numbering according to Kabat et al. [24]).