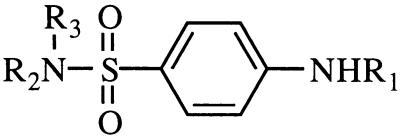
TABLE 2.
Structures and activities of various reexamined sulfanilamides
| Compoundf | Substituent at position:
|
IC50 (μM) | ||
|---|---|---|---|---|
| R1 | R2 | R3 | ||
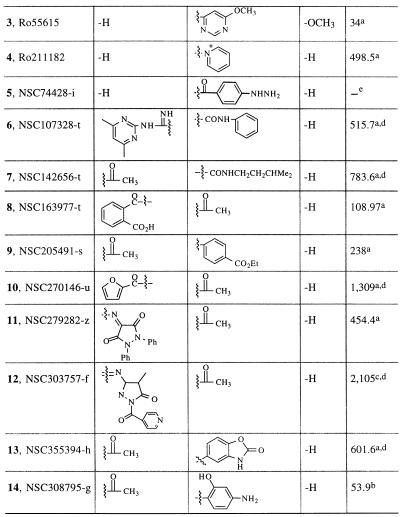 | ||||
Inhibition was tested at concentrations of 100 and 500 μM, and the resulting data were pooled with earlier inhibition data to generate IC50s as reported previously (7).
Inhibition was tested at a concentration of 100 μM, and the resulting data were pooled with earlier inhibition data to generate IC50s as reported previously (7).
Inhibition was tested at a concentration of 500 μM, and the resulting data were pooled with earlier inhibition data to generate IC50s as reported previously (7).
The calculated IC50 is outside of the experimental range.
—, a negative correlation between the drug concentration and inhibition was noted.
Numbers in boldface are compound numbers.