Figure 1. X-TOLE Trial Design and Patient Disposition.
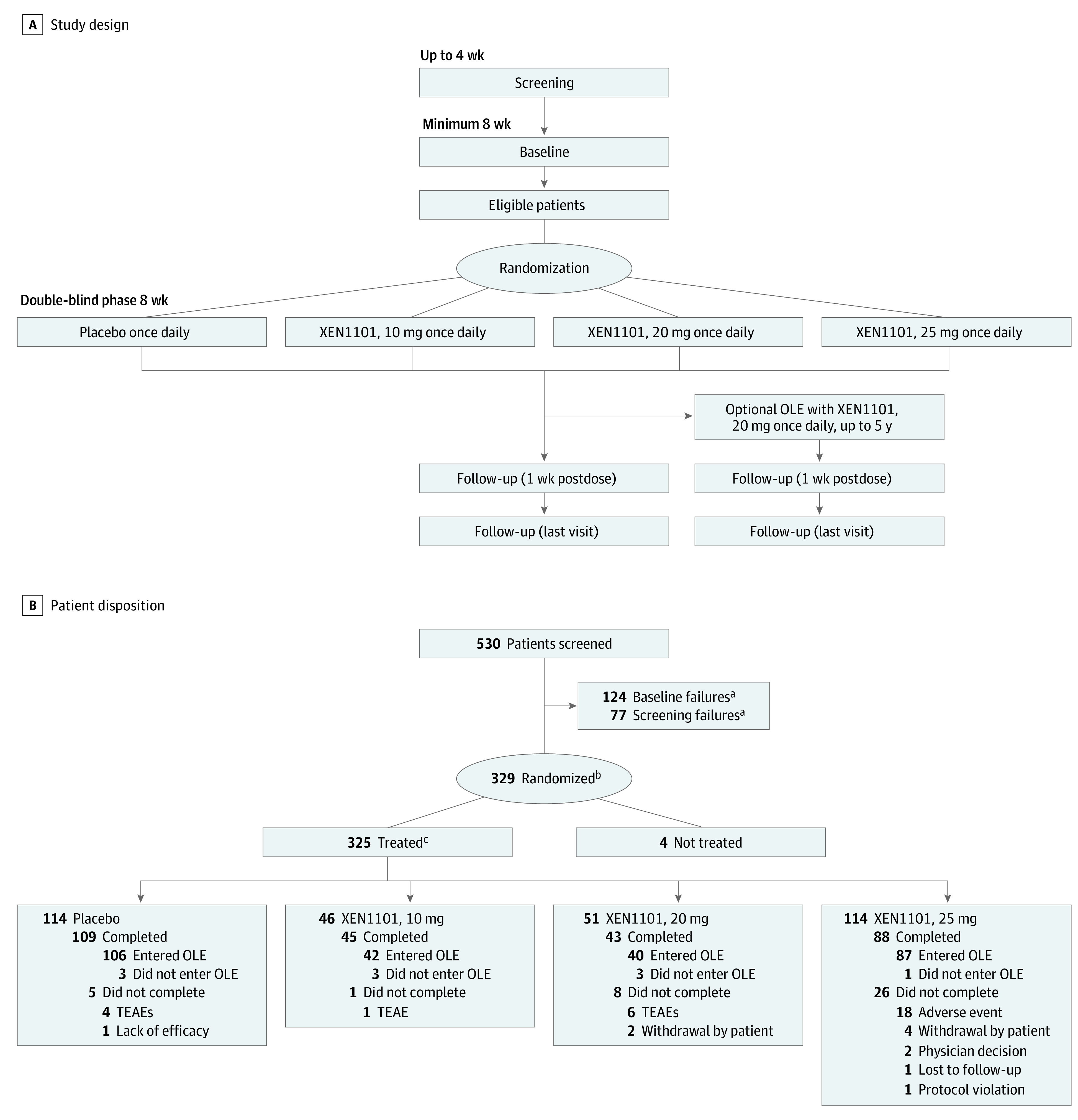
A, X-TOLE trial design, with 2:1:1:2 randomization. B, Patient disposition. Screening included all patients who signed informed consent and were entered into the clinical database. All doses were administered as a once-daily capsule with food, and titration was not required. OLE indicates open-label extension; TEAE, treatment-emergent adverse event.
aScreening failures and patients who did not enter baseline for any other reason.
bAll patients who were provided a treatment assignment and recorded in the interactive response technology database, regardless of whether treatment was used.
cPatients in the safety population.
