Abstract
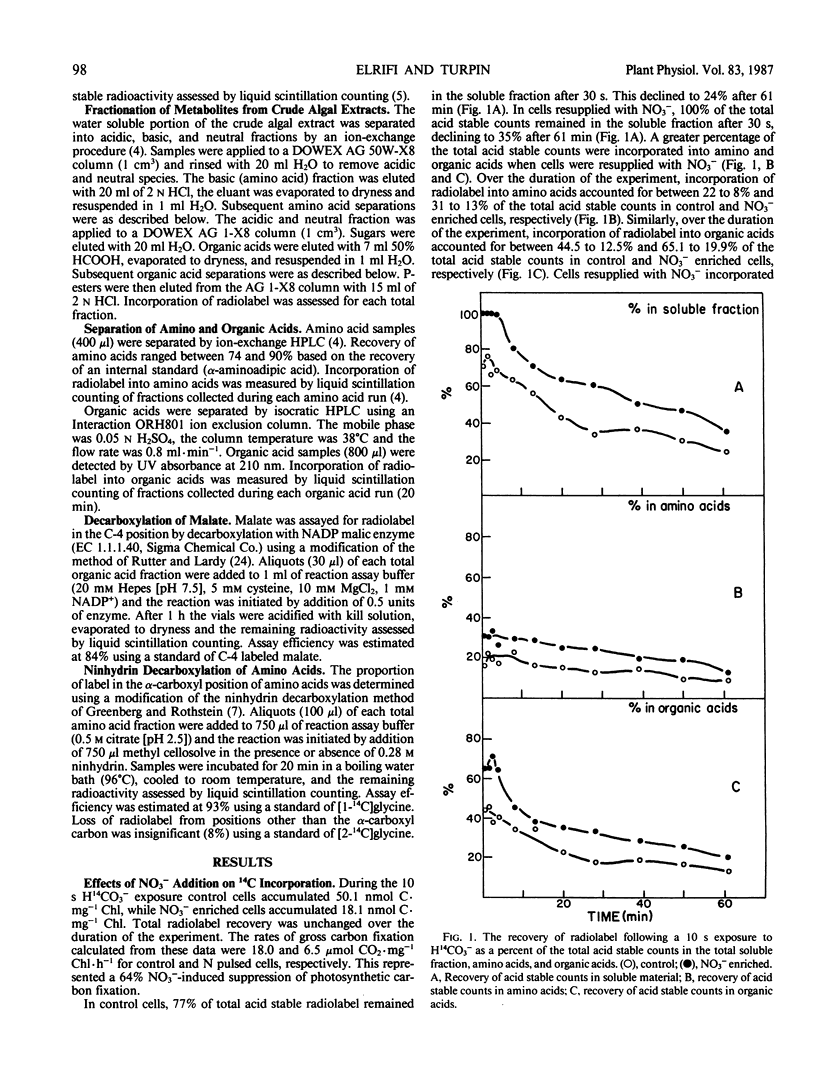
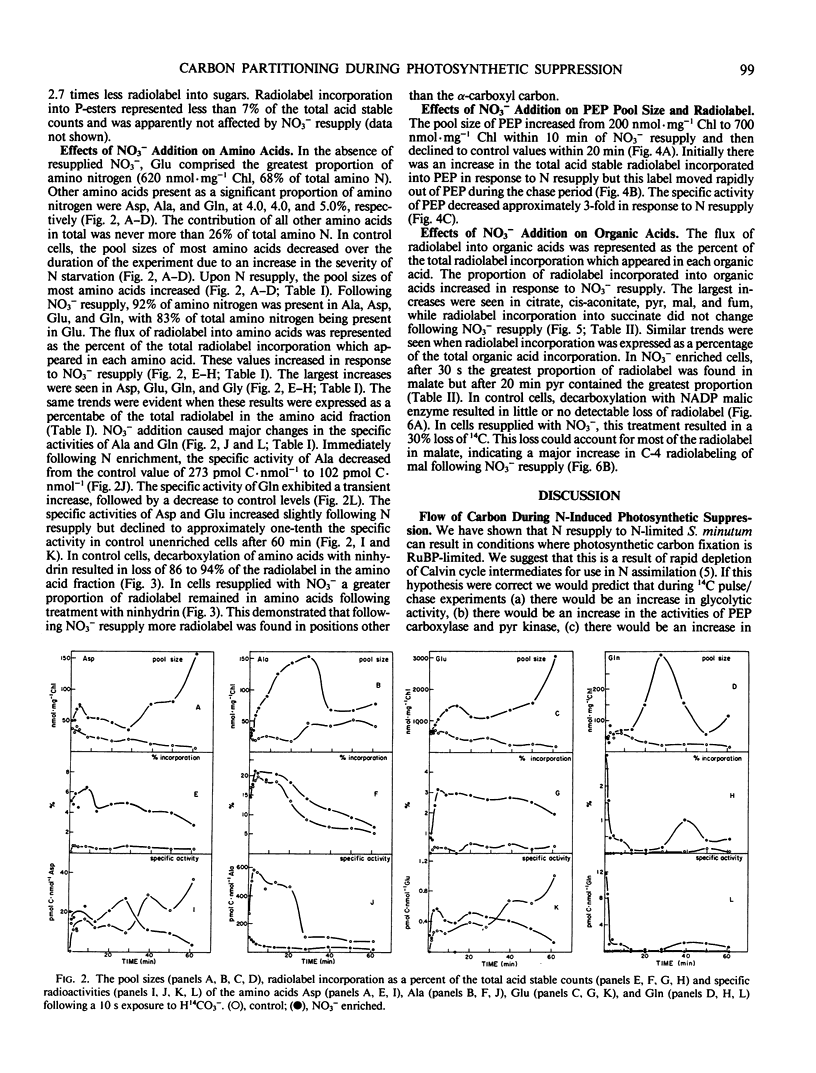
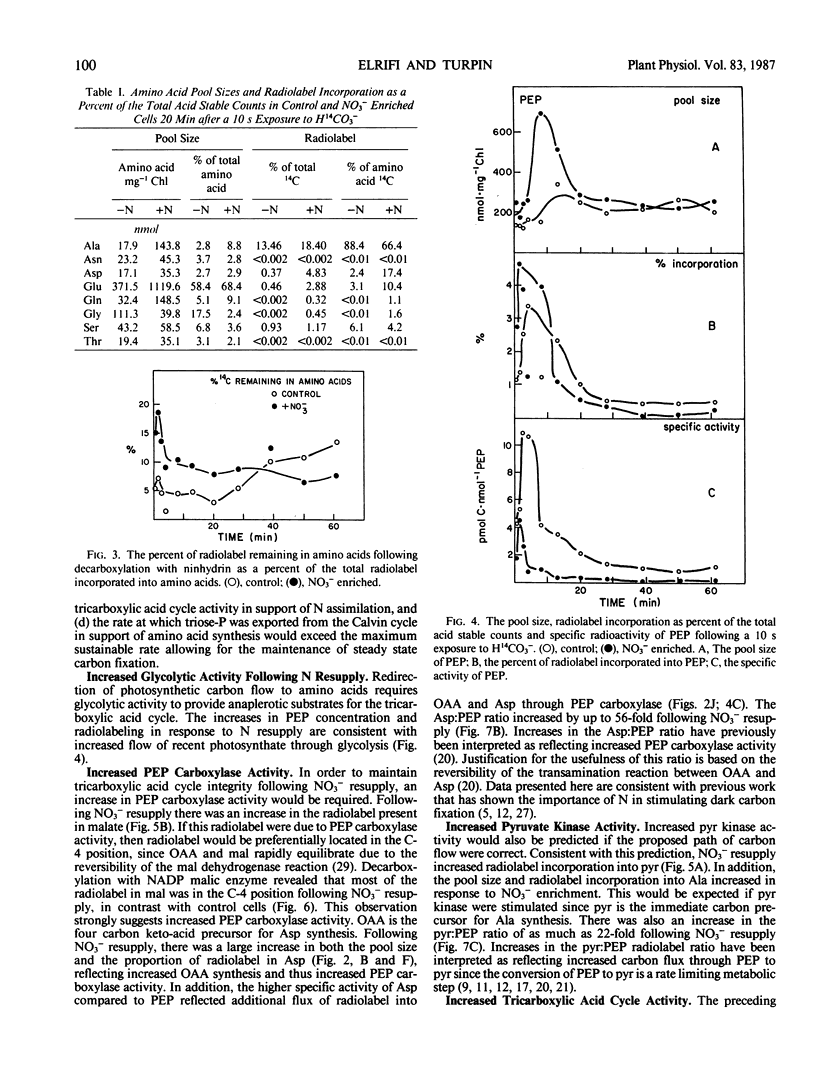
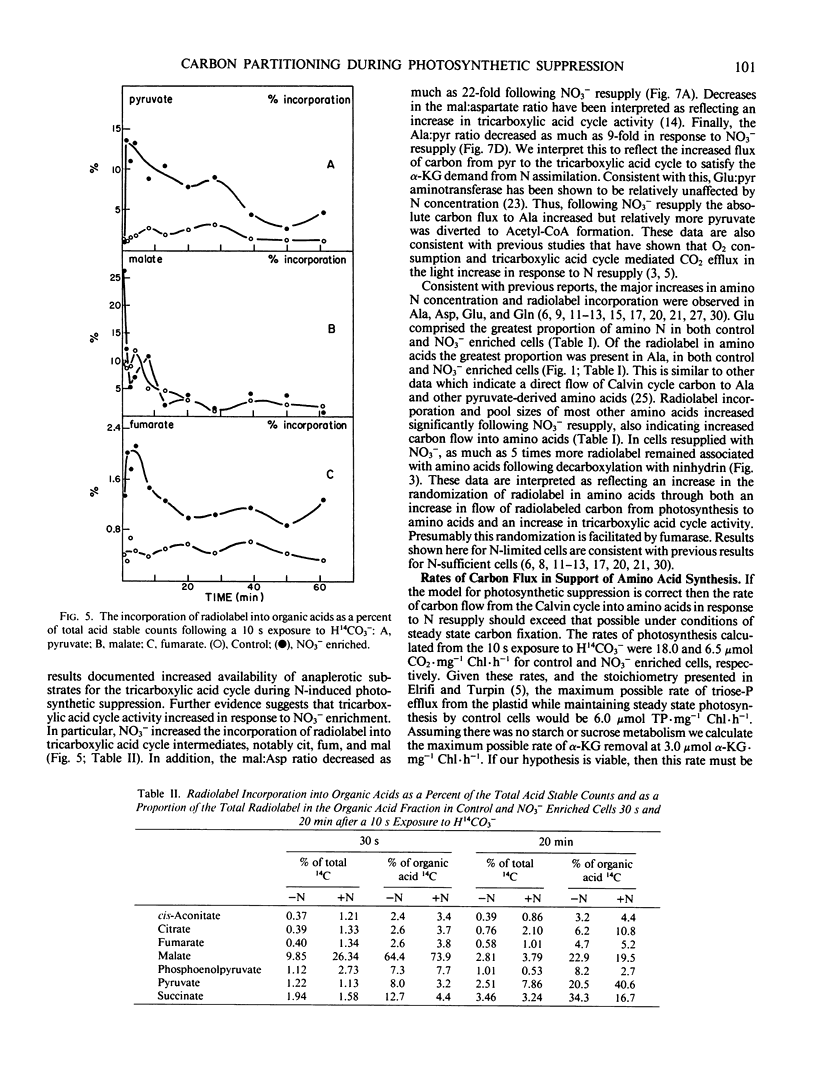
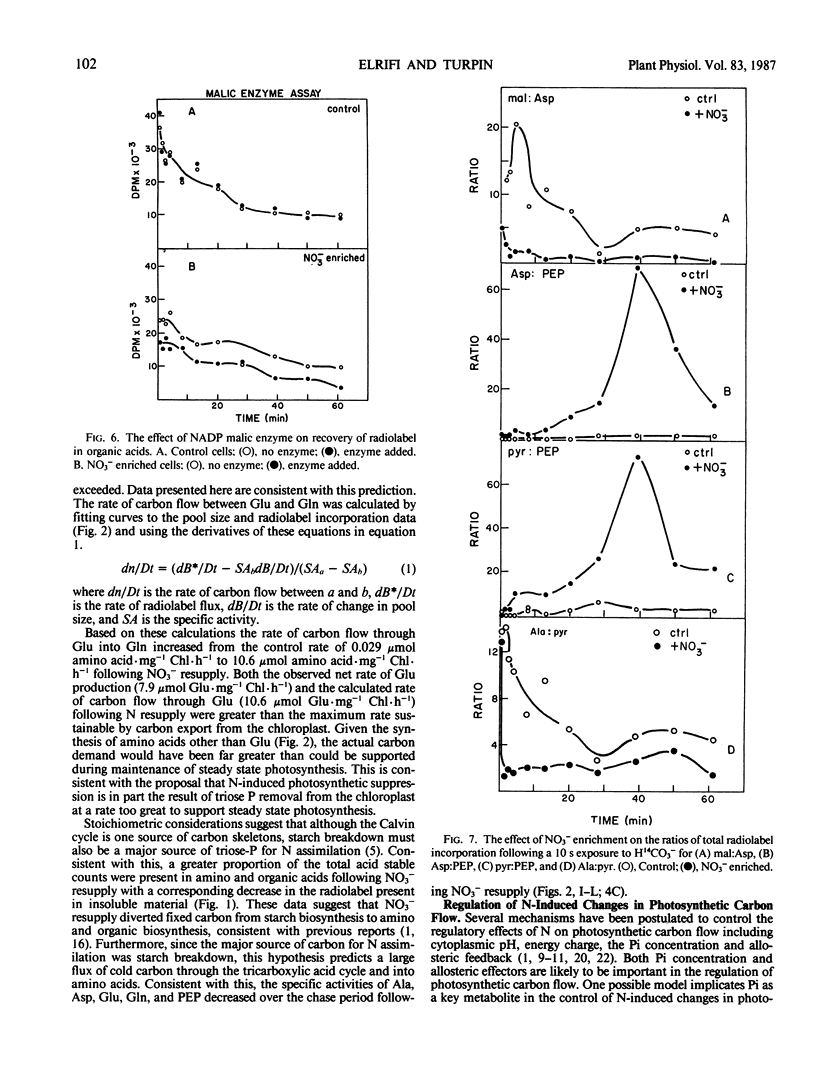
Nitrate addition to nitrate-limited cultures of Selenastrum minutum Naeg. Collins (Chlorophyta) resulted in a 70% suppression of photosynthetic carbon fixation. In 14CO2 pulse/chase experiments nitrate resupply increased radiolabel incorporation into amino and organic acids and decreased radiolabel incorporation into insoluble material. Nitrate resupply increased the concentration of phosphoenolpyruvate and increased the radiolabeling of phosphoenolpyruvate, pyruvate and tricarboxylic acid cycle intermediates, notably citrate, fumarate, and malate. Furthermore, nitrate also increased the pool sizes and radiolabeling of most amino acids, with alanine, aspartate, glutamate, and glutamine showing the largest changes. Nitrate resupply increased the proportion of radiolabel in the C-4 position of malate and increased the ratios of radiolabel in aspartate to phosphoenolpyruvate and in pyruvate to phosphoenolpyruvate, indicative of increased phosphoenolpyruvate carboxylase and pyruvate kinase activities. Analysis of these data showed that the rate of carbon flow through glutamate (10.6 μmoles glutamate per milligram chlorophyll per hour) and the rate of net glutamate production (7.9 μmoles glutamate per milligram chlorophyll per hour) were both greater than the maximum rate of carbon export from the Calvin cycle which could be maintained during steady state photosynthesis. These results are consistent with the hypothesis that nitrogen resupply to nitrogen-limited microalgae results in a transient suppression of photosynthetic carbon fixation due, in part, to the severity of competition for carbon skeletons between the Calvin cycle and nitrogen assimilation (IR Elrifi, DH Turpin 1986 Plant Physiol 81: 273-279).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASSHAM J. A., KIRK M. PHOTOSYNTHESIS OF AMINO ACIDS. Biochim Biophys Acta. 1964 Sep 4;90:553–562. doi: 10.1016/0304-4165(64)90234-x. [DOI] [PubMed] [Google Scholar]
- Birch D. G., Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum: II. Effects of NO(3) and NH(4) Addition to CO(2) Efflux in the Light. Plant Physiol. 1986 Nov;82(3):708–712. doi: 10.1104/pp.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1986 May;81(1):273–279. doi: 10.1104/pp.81.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Larsen P. O., Cornwell K. L., Gee S. L., Bassham J. A. Amino Acid Synthesis in Photosynthesizing Spinach Cells : EFFECTS OF AMMONIA ON POOL SIZES AND RATES OF LABELING FROM CO(2). Plant Physiol. 1981 Aug;68(2):292–299. doi: 10.1104/pp.68.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie A. C., Codd G. A., Stewart W. D. The incorporation of nitrogen into products of recent photosynthesis in Anabaena cylindrica Lemm. Arch Microbiol. 1976 Feb;107(1):15–24. doi: 10.1007/BF00427862. [DOI] [PubMed] [Google Scholar]
- Lawyer A. L., Cornwell K. L., Larsen P. O., Bassham J. A. Effects of carbon dioxide and oxygen on the regulation of photosynthetic carbon metabolism by ammonia in spinach mesophyll cells. Plant Physiol. 1981 Dec;68(6):1231–1236. doi: 10.1104/pp.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A. H., Gnanam A. A possible mechanism of ammonium ion regulation of photosynthetic carbon flow in higher plants. Plant Physiol. 1979 Aug;64(2):263–268. doi: 10.1104/pp.64.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori M., Hattori A. Transient change in the ATP pool of Anabaena cylindrica associated with ammonia assimilation. Arch Microbiol. 1978 Apr 27;117(1):17–20. doi: 10.1007/BF00689345. [DOI] [PubMed] [Google Scholar]
- Platt S. G. Ammonia regulation of carbon metabolism in photosynthesizing leaf discs. Plant Physiol. 1977 Nov;60(5):739–742. doi: 10.1104/pp.60.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTTER W. J., LARDY H. A. Purification and properties of pigeon liver malic enzyme. J Biol Chem. 1958 Aug;233(2):374–382. [PubMed] [Google Scholar]
- Schulze-Siebert D., Heineke D., Scharf H., Schultz G. Pyruvate-Derived Amino Acids in Spinach Chloroplasts : Synthesis and Regulation during Photosynthetic Carbon Metabolism. Plant Physiol. 1984 Oct;76(2):465–471. doi: 10.1104/pp.76.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]


