Fig. 1.
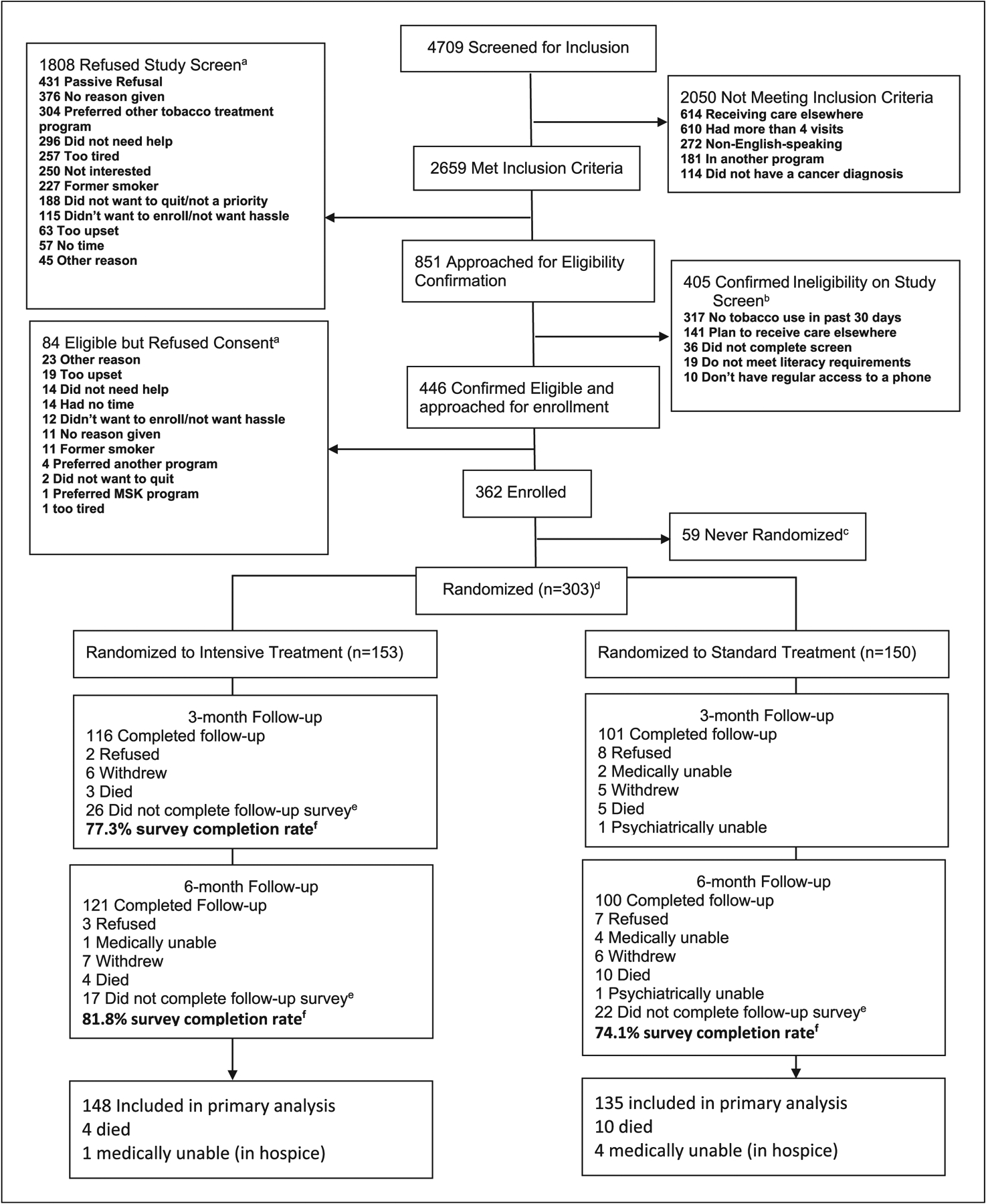
Flowchart of enrollment and intervention to test the effectiveness of two models of tobacco treatment integrated into cancer are at diagnosis. Trial, November 2013 to July 2017. aPatients could give multiple reasons for refusal. The research assistant categorized the reasons of refusal that patients offered according to options available on the study screening tool; those reasons that did not fit into one of these predefined categories were discussed with the team to determine fit with existing categories or establishment of new categories. bMultiple reasons for ineligibility could have been indicated on the screener. As such, the number of reasons exceed the number of patients ineligible. cThose who were never randomized were those who signed a consent form but did not complete a counseling session and were thus not randomized to a treatment group. Reasons included participants who were not able to be reached by the study counselor, participants who withdrew citing other cancer care demands, and participants who became ineligible over time. dA computer was used to randomize participants. Blocks of 6 were used for treatment assignment and were stratified by cancer center clinic and study site. eThose who did not complete follow-up survey include those who were not able to be reached at all to complete the follow-up assessment.fFollow-up survey completion rate = completed/completed+ refused+withdrew+did not complete follow-up survey. Participants who were deceased or medically ineligible (e.g., in inpatient hospice or psychiatrically impaired) at follow-up were not included in the final outcome analyses (n = 5 intensive treatment and n = 15 standard treatment). Thus, for the intensive treatment, the denominator = 148, and for the standard treatment, the denominator = 135.
