Abstract
The tumours of head and neck district are around 3% of all malignancies and squamous cell carcinoma is the most frequent histotype, with rapid increase during the last two decades because of the increment of the infection due to human papilloma virus (HPV). Even if the gold standard for the diagnosis is histological examination, including the detection of viral DNA and transcription products, imaging plays a fundamental role in the detection and staging of HPV + tumours, in order to assess the primary tumour, to establish the extent of disease and for follow-up. The main diagnostic tools are Computed Tomography (CT), Positron Emission Tomography-Computed Tomography (PET-CT) and Magnetic Resonance Imaging (MRI), but also Ultrasound (US) and the use of innovative techniques such as Radiomics have an important role. Aim of our review is to illustrate the main imaging features of HPV + tumours of the oropharynx, in US, CT and MRI imaging. In particular, we will outline the main limitations and strengths of the various imaging techniques, the main uses in the diagnosis, staging and follow-up of disease and the fundamental differential diagnoses of this type of tumour. Finally, we will focus on the innovative technique of texture analysis, which is increasingly gaining importance as a diagnostic tool in aid of the radiologist.
Keywords: Oropharynx, HPV, Head and Neck, Oncology, Magnetic resonance imaging, Ultrasound, Computed tomography, Radiomics
Introduction
The tumours of head and neck district are around 3% of all malignancies and squamous cell carcinoma is the most frequent histotype [1–4]. The incidence of these subtypes of tumour has been rapidly increasing during the last two decades because of the increment of the infection due to human papilloma virus (HPV), cause of tumours that mainly develop into lingual and palatine tonsils tissue [5]. Regarding HPV-positive (HPV+) squamous cell tumour, it is most commonly found in males with age of around 40–60 years. They are not related with tobacco exposure or smoking, or alcohol consumption. The most frequent genotypes involved are genotype 16 and 18, that are considered therefore oncogenic [6].
The diagnosis of OPSCC is based on pan-endoscopy with biopsies of the primary lesion [7]. When traditional endoscopic techniques might fail to identify the primitive lesion, the better visualization and freedom of motion of trans-oral robotic surgery (TORS) techniques might also help with biopsy [8, 9]. The gold standard for the diagnosis is histological examination, including the detection of viral DNA and transcription products [10].
On imaging HPV + tumours differ from HPV-negative (HPV-) ones [11]. Imaging is mandatory in order to permit the correct extension of disease, to evaluate lymph-nodal involvement and the presence of distant metastasis.
Ultrasound is used for the evaluation of suspected laterocervical lymphnodal metastasis. HPV + tumours usually show cystic and necrotic nodal metastasis, differently from HPV- ones [12]. At computed tomography (CT) and magnetic resonance imaging (MRI) HPV + primary tumours often detect well-defined lesions with exophytic growth with vivid homogeneous enhancement [12–14]. The superior soft-tissue contrast of MRI imaging allows for easier detection of nodal metastases and better definition of tumour extension, margins, and vascular or nervous involvement [15–19].
Also, innovative new techniques are also increasingly being used in the evaluation of this type of tumour, including MRI perfusion imaging and texture analysis.
The aim of this review is to illustrate the main imaging features of HPV + tumours of the oropharynx, in US, CT and MRI imaging. In particular, we will outline the main limitations and strengths of the various imaging techniques, the main uses in the diagnosis, staging and follow-up of disease and the fundamental differential diagnoses of this type of tumour. Finally, we will focus on the innovative technique of texture analysis, which is increasingly gaining importance as a diagnostic tool in aid of the radiologist.
Diagnostic role of ultrasound
While the inherent limits of ultrasound (US) imaging don’t generally allow for the evaluation of primary site HPV-related cancer, its availability, relative low-cost and its great definition for the study of superficial structures make it ideal to investigate suspicious lymph node swelling in the head and neck district [20–23]. Thus, the main role played by US in the study of HPV-related cancer consists in the identification of potential nodal metastatic disease in tumors of unknown primary origin [11, 20–22, 24].
While no specific nodal station in related to HPV disease as is, it is true that metastatic drainage in the head and neck is district specific [21, 22, 25, 26]. Thus, a lymph node with anomalous features on ultrasound might be indicative of the presence of a primary tumor which location might be inferred by the radiologist with sufficient knowledge of the pattern of distribution of metastatic nodes.
Cancers of the oropharynx, hypopharynx and larynx tend to metastasize more frequently to the internal jugular chain (leves II-III-IV), while upper cervical and submandibular chains (levels Ia-Ib and IIa) are more frequently related to oral cancers [21, 22]. While HPV-positive nasopharyngeal carcinomas are rarer overall, it is important to note that these neoplasms tend to metastasize to the upper cervical or posterior triangle stations.
Size
Size is considered an indirect indicator of malignancy.
Optimal cut-off values for the minimal diameter have been identified as 10 mm cervical nodes on an axial plane [27]. It is important to note that size alone cannot be used to evaluate possible metastatic lymph node, and it should also be kept in mind that upper neck nodes are usually bigger than lower neck ones.
In addition, size evaluation over time can be useful for patients that have already been diagnosed with a primary head and neck tumour.
Shape
Normal or inflammatory nodes usually appear as oval or flat in shape. Metastatic lymph nodes tend to be round in shape. Borders of malignant nodes are typically sharper and better defined from surrounding tissues than normal on ultrasound [21, 28]. A possible explanation of this phenomenon is the increased acoustic impedance difference between node and tissue that is a byproduct of reduced fatty infiltration and the presence of tumor cells that have replaced lymphoid tissue inside the node itself [21].
Echogenicity
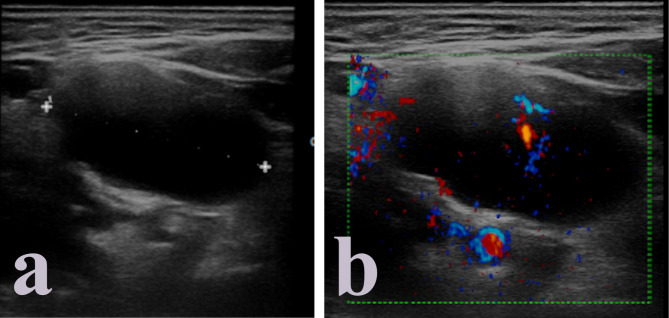
HPV-related metastatic lymph nodes tend to be hypoechoic than adjacent muscle tissue. Usually, no hyperechoic hilus can be detected even though it might still be present in the earlier phases of the process of tumoral invasion of the node. A typical sign of squamous cell HPV-related carcinoma is the development of echolucent cystic necrosis (Fig. 1), which is a feature common to every imaging modality (Table 1). It should be noted that tubercular nodes and metastases form papillary thyroid cancer can also present cystic necrosis as a late alteration to nodal structure, as well as benign cysts of the neck such as branchial cleft cysts. Hyperechoic spots, sign of coagulation necrosis, are rare, as well as intranodal calcifications, which are more likely to appear as a consequence of chemotherapy.
Fig. 1.
Echolucent cystic necrosis in HPV + metastatic cervical lymph node
Table 1.
Elements of differential diagnosis between HPV + and HPV- oropharyngeal cancer across different imaging techniques
| Imaging technique | HPV+ | HPV- |
|---|---|---|
| Ultrasound | ||
| Primary lesion | - | - |
| Metastatic lymph nodes |
Size > 8 mm (9 mm subdigastric IIa) Round shape |
|
| Hypoechoic cystic necrosis |
Hyperechoic spots (coagulation necrosis)- rare Intranodal calcification |
|
| CT | ||
| Primary lesion |
Smaller Exophytic growth Well defined borders |
Bigger Ill-defined borders Grows by invading submucosa and adjacent muscle tissue Necrosis Ulceration |
| Metastatic lymph nodes |
Cystic necrosis Well-defined borders Clustering |
Extra-nodal extension Ill-defined borders Matting |
| PET-CT | ||
| Higher SUVmax | Lower SUVmax | |
| Conventional MRI | ||
| Primary lesion | Same elements as CT but better overall definition | |
| Metastatic lymph node | ||
| DWI | ||
| Primary lesion |
Lower ADC and Dt; Leptokurtic Right skewed ADC histogram |
Higher ADC and Dt; Symmetric Normally distributed ADC histogram |
| Metastatic lymph node | ||
| IVIM | No significant difference in D*, skewness, kurtosis | |
| DKI | ||
| DCE-MRI | ||
| Primary lesion | Inconstant lower values of ktrans | - |
| Metastatic lymph node | ||
Inflammatory edema of surrounding tissue might be present around all metastatic nodes, regardless of HPV correlation. Lymph node matting is rare.
Color-doppler
Vascular pattern of metastatic nodes tends to be peripheral, as no clear hilar vascularization can be identified. In the presence of cystic necrosis, however, it is possible to observe avascular nodes due to vase destruction.
Additional use
In addition to the imaging features that might help in the evaluation of nodal metastases, it is also important to remember the role played by US in ultrasound-guided fine needle aspiration cytology (FNAC) for the definitive diagnoses. The employment of ultrasound to provide more accurate information can be used both for diagnosis and follow-up.
Diagnostic role of computed tomography
Along standard MRI, CT imaging has become one of the fundamental imaging techniques for both diagnosis and staging of head and neck cancer, regardless of HPV status.
Diagnosis
CT plays a vital role in the definition of primary tumor in the head and neck region. To this end, CT has been employed both to better define the size and relation to contiguous tissues and structures of a suspect lesion found on clinical examination or during laryngoscopy and to study the head and neck district in search of a primary tumor of unknown origin when faced with suspicious lymph node swelling. To these ends, contrast-enhanced CT of the head and neck region is fundamental to both identify the lesion and to better evaluate its extension to surrounding tissues.
While definitive diagnosis of HPV related disease can only be proven through histology, a certain amount of imaging features can help the radiologist to infer the HPV status of a tumoral mass. In addition to that, a number of prognostic biomarkers can be evaluated during staging, allowing for a better definition of oncologic risk.
Imaging elements of differential diagnosis of HPV-related disease on CT imaging
HPV-related cancers of the head and neck district tend to present certain features that can help the radiologist in their characterization (Table 1).
When it comes to non-contrast imaging, among morphological and topographical differences between HPV + and HPV- disease can be found the usually smaller overall size of HPV + lesions and their exophytic growth [13, 29].
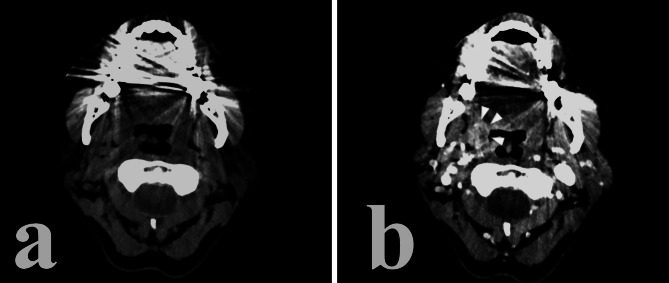
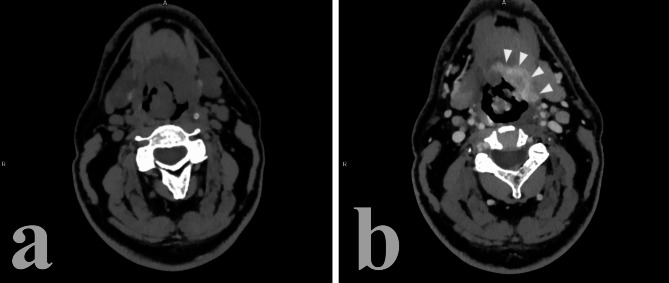
On post-contrast CT, HPV-related cancer tends to show a stronger enhancement as opposed to HPV- disease which is usually less enhanced [30, 31] (Figs. 2 and 3). It should be noted that this difference is very subtle and not always present [13].
Fig. 2.
HPV + right tonsil cancer. Notice the relatively high post-contrast enhancement of the lesion, its well-defined borders and exophitic growth
Fig. 3.
HPV- cancer of the base of the tongue and lower tonsillar pole. Notice the relatively low post-contrast enhancement, ill-defined borders and infiltration of adjacent structures such as the oral pelvis
HPV-related cancer usually presents itself as an exophytic lesion with well-defined borders and little to no invasion of the submucosa or of the adjacent tissues, such as muscle [13, 29–32] (Fig. 2). Non-HPV lesions, on the other hand tend to show borders that are ill-defined and are more likely to develop invasion of adjacent tissue and of the submucosa [31] (Fig. 3). Ancillary features such as ulceration of the mass or necrosis can be shown in a subset of HPV + primary lesions, but they tend to be more characteristic of HPV- cancer [29, 30].
Aside from considerations that the radiologist can make on primary lesion, there are a number of features to be investigated on metastatic lymph nodes as well. Most of such considerations have already been reported in the ultrasound section of this paper and, as such, will only be cited briefly.
No specific nodal level can be correlated directly to HPV + disease, as lesions can equally metastasize to each level of the head and neck district regardless of HPV status [13, 29, 31, 33]. Nodal level involvement depends on the site of the primary tumor [21, 22]. Retro-pharyngeal metastatic nodes can be involved in both HPV + and HPV- disease equally, as happens with contralateral node involvement [29, 33].
As previously stated, cystic necrosis of lymph nodes is one of the defining features of HPV disease [21, 22, 29, 33]. Cystic necrosis can be identified on CT imaging as a regular, usually oval or circular in shape, area of hypoattenuation inside the lymph node which may show peripheral contrast enhancement on post-contrast imaging [13, 15, 34]. While lymph nodes tend to show clustering, it should be noted that in the case of HPV + disease matting of nodes is less likely to happen [29, 33].
Similarly to primary tumors, the borders of the swollen metastatic lymph node are more likely to be well-defined, with radiologic extra nodal extension (rENE) of adjacent structures being rarer in HPV + disease [13, 33].
Staging and prognosis
CT imaging is most commonly used to stage head and neck cancer, regardless of HPV status [31, 35–37].
CT imaging of primary lesion can be used, with or without the involvement of MRI, to determine tumor size and invasion of adjacent tissue [31, 36–38]. CT can also be used to better evaluate involvement of cortical bone structures adjacent to the lesion thanks to its superior power in the definition of spatial bone and cartilage anatomy, especially when the skull base, sinuses, mandibular bone and maxilla are involved [39, 40] which is fundamental to determine possibilities of surgical approach and overall for T staging [13, 36, 37].
In addition to that, nodal involvement can be evaluated via CT in order to define N staging. While MRI can be used to detect metastatic lymph nodes, CT tends to be more reliable and, to this extent, better overall for N staging [40].
Finally, the employment of High-Resolution CT (HRCT) of the chest allows for a better evaluation of possible metastases to the lung and is thus used for M staging.
Diagnostic role of PET-CT
Positron emission tomography-computed tomography is a technique that combines the precise anatomical definition of a CT scan with information regarding the metabolic activity of primary tumor and metastatic sites. As such PET-CT plays a fundamental role in both staging and post treatment surveillance of OPSCC, regardless of HPV status [41].
Fusion CT images produces by PET-CT may sometimes provide a better definition of primary site tumors, especially in those sites that are notoriously harder to study through other techniques (oral cavity, tongue etc.) especially with smaller lesions that might have been easy to spot on clinical examination but might be too small to show significant contrast enhancement to help differentiate from surrounding tissue [42]. This is especially useful for tumors of unknown primary site, that might be brought to the clinician’s attention through nodal involvement before the primary lesion becomes symptomatic [41, 42]. In these situations PET-CT techniques in addition to endoscopic or TORS techniques might facilitate primary site localization and have been shown to be in fact superior to CT imaging alone and comparable to DWI MRI [8, 43, 44].
When it comes to HPV-correlation, multiple studies have shown that HPV + cancers tend to present themselves with higher SUVmax values when compared to HPV- tumors [41, 45]. As such PET-CT can be another instrument to help infer HPV status in oropharyngeal cancer.
Diagnostic role of MRI
Conventional T1-weighted and T2-weighted MRI
Diagnosis
While conventional, anatomical, MRI yields results similar to CT in terms of employment in the diagnostic stage of head and neck cancer [29, 40], as well as similar findings in the differential diagnosis of HPV status, it should be noted that the arguably superior definition when it comes to soft-tissue contrast [46–48] and to possibility to employ specific sequences to better highlight anatomical structures, such as fat suppression sequences, can allow for better visualization of primary lesion anatomy and its relationship with surrounding tissue [29, 46–52].
Imaging elements of differential diagnosis of HPV-related disease on conventional MRI imaging
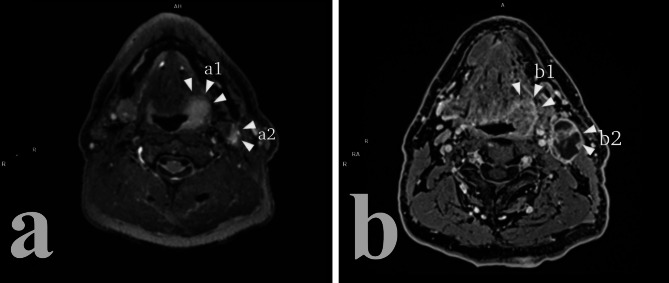
As stated above, elements of differential diagnosis of HPV status in head and neck cancer are comparable between CT and MRI and, as a such, will only be briefly repeated (Table 1). MRI imaging of HPV + primary tumor tends to show overall smaller lesions, exophytic, with less submucosal or adjacent tissue spreading overall [13, 29, 53, 54] (Figs. 4 and 5). Ulceration and necrosis are also less common then in HPV- tumors [13, 54].
Fig. 4.
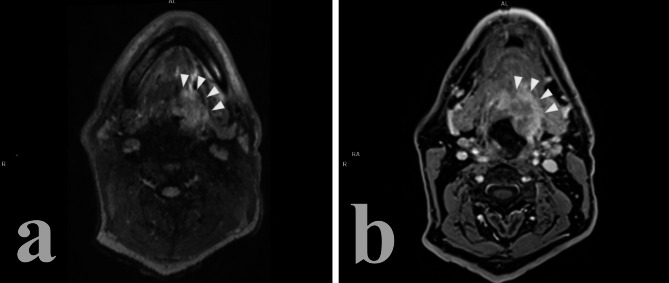
HPV + oropharyngeal cancer (a1, b1) with adjacent cystic metastatic lymph node (a2, b2). (a) T2w; (b) post-contrast T1w. The relative higher soft tissue resolution of MRI highlights the contained nature of the lesion and its well-defined borders
Fig. 5.
HPV- cancer of the base of the tongue. (a) T2w; (b) Post-contrast T1w. Notice the ill-defined borders and the locoregional invasion of adjacent tissue
No significant difference seems to be present after contrast administration between HPV + and HPV- lesions [13, 29, 53–55].
When it comes to nodal involvement, the same consideration that were made for CT are valid for MRI, with metastasized lymph nodes that tend to develop cystic necrosis (focal area of homogenous high intensity on T2-weighted imaging with or without peripheral contrast-enhancement on post-contrast T1-weighted imaging), to form clusters without signs of matting, with well-defined borders and little to no radiologic extra-nodal extension (rENE) [13, 24, 56–58] (Fig. 4).
Staging and prognosis
Due to its superior accuracy and contrast resolution, MRI is often employed for the local staging of tumoral lesions, and local extension to adjacent tissues [26, 52, 59].
Locoregional nodal involvement can also be studied with MRI techniques with high sensitivity and specificity [59].
Functional MRI
In these recent years, the employment of functional MRI modalities alongside conventional morphological imaging techniques has been steadily rising in the study of head and neck cancer [52, 54]. Diffusion weighted imaging allows to infer information on tumor cellularity and ultrastructure [52, 54, 60–63], whereas Dynamic contrast-enhanced MRI (DCE-MRI) allows for the indirect evaluation of the kinetic of distribution of contrast agent and, thus, of the nature of tumoral vascularization [48, 51, 64, 65].
Diffusion weighted imaging (DWI)
When it comes to DWI, it has been reported that HPV + lesions tend to overall show lesser values in both tissue diffusion coefficient (Dt) and apparent diffusion coefficient (ADC) when compared to HPV- tumors [25, 48, 54, 56, 66, 67] (Fig. 6). This difference seems to stem from the different architectural heterogeneity that the two neoplasms have, with HPV- cancer showing higher microstructural cellularity [66].
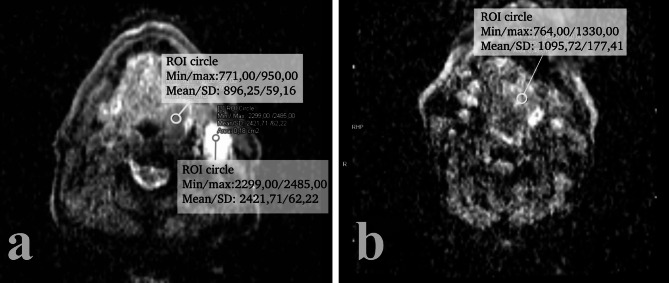
Fig. 6.
ADC map of HPV+ (a) and HPV- (b) oropharyngeal cancer. Notice the lower mean ADC value of the HPV + lesion (896,25 vs. 1095,72)
It should also be noted that many authors [66, 67] have pointed out that, while most ADC maps are obtained monoexponential calculation with 2 b values (most often 0-800/1000), the choice of b value affects the ability of DWI to discern HPV status, the “optimal” choice being 0-1000 [67]. To this end, pixel-based calculation of ADC maps in like parametric maps and ADC histograms have been shown to be able to asses HPV status [66, 67]. Studies seem to suggest that HPV- cancer shows a symmetric normal distribution of ADC histograms, correlating with it microstructural heterogeneity, while HPV + cancer seems to show leptokurtic skewed right ADC histograms [66].
The lower values in DWI seem to not only be typical of primary site HPV + disease, but also for metastatic HPV + lymph nodes which show overall lower ADC and Dt than their HPV- counterpart [25, 54, 56].
It should also be noted that other forms of diffusion weighted imaging such as intra-voxel incoherent motion (IVIM) with evaluation of D* and diffusion kurtosis imaging (DKI) with evaluation of skewness and kurtosis don’t seem to produce any significant element of differential diagnosis for HPV status [48, 54] though some studies, such as Salzillo et al. [30], report finding that HPV + primary site tumors tend to have higher kurtosis and skewness values and lower values of D* (Table 1). Nonetheless, the value of these imaging modalities in the definition of HPV status seems to be a topic for further studies.
Dynamic contrast-enhanced imaging (DCE-MRI)
The role played by DCE-PWI in the seems to be unclear, though higher values of Ktrans parameter tend to be associated with HPV + lesions, this association seems to be inconstant and not significative enough to provide sufficient evidence in most cases [30, 68–70] (Figs. 7 and 8).
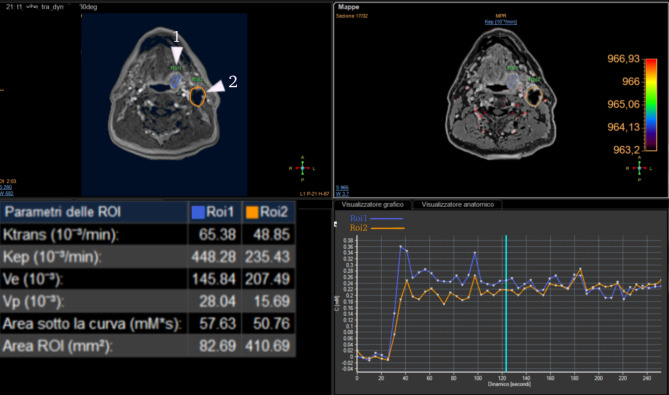
Fig. 7.
Perfusion curve of HPV + oropharyngeal cancer (1) and metastatic lymph node (2)
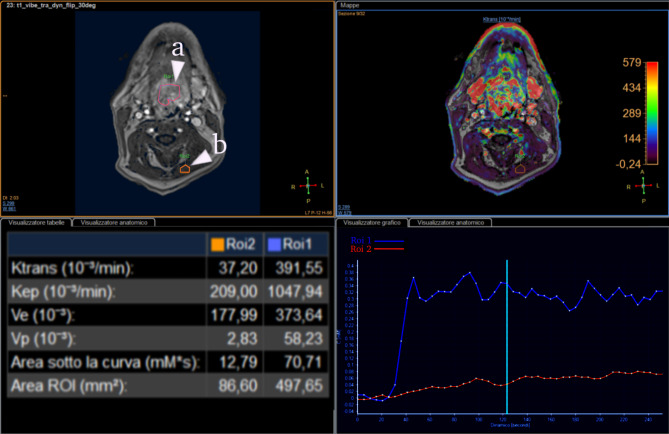
Fig. 8.
Perfusion curve of HPV- oropharyngeal cancer (a, ROI1) compared to muscle (b, ROI2). Notice the lower Ktrans value when compared to the one in Fig. 7
Role of texture analysis
Radiomics with texture analysis is a new technique that in recent years is gaining lot of visibility for its capacity of characterize tissue heterogeneity in order to identify and to differentiate structures such as neoplasms or organs, using a region of interest (ROI) and the extraction of features. It can be applied on CT, MRI and also on positron emission tomography (PET-CT) [71–78]. It consisted in measuring the spatial distribution of pixel values to retrieve structural information not perceptible by human eye. It is based on the extraction of first, second and higher-order parameters. The first order uses the histogram to study the gray-level distribution frequency within the ROI in order to evaluate the single pixel and not its interactions with adjacent pixels. The second order evaluates how often the intensity of one pixel has a specific relationship to that of another pixel through gray-level co-occurrence matrix (GLCM) measurements. A further way to derive second-order parameters is the gray-level run length matrix (GLRLM) that analyses consecutive pixels with the same intensity in a defined direction. The higher orders assess differences between pixels and voxels in the context of the entire ROI using a neighborhood gray-tone-difference matrix (NGTDM) by identifying variations within the examined space in gray-level intensity [79–84]. The use of texture analysis is becoming increasingly used as an important tool in support for the radiologist in the diagnosis and assessment of disease recurrence-persistence after surgery or radiochemoteraphy. It can also be used to evaluate prognosis and in particular in the early differentiation between HPV + or HPV- neoplasms and also to detect and evaluate lymph node neck metastases.
Diagnosis
The use of radiomics applied on CT and MRI images has becoming increasingly interesting both for diagnosis and evaluation of pre-therapy HPV + oropharyngeal cancer. In fact, lot of studies are evaluating whether these tools can be able to characterize tumour histotypes [85–92] and in particular HPV + from HPV-, because of the great difference in prognosis and response to therapy of these two different neoplasms [93–96]. For this reason, several studies were conducted in order to differentiate and evaluate preliminarily the HPV status of a lesion both on CT and on MRI imaging. The first order features included shape features, both descriptors of the two or three-dimensional size and shape of the ROI. In the study by Choi et al. [86], for example, CT images of 86 untreated patients were analyzed and it was found how lower values of a specific feature (spherical disproportion) was associated to HPV positivity, due to their more regular shape. Similar results were also found in the study by Yu et al. [87]. Also, Bos et al. [97] based their study on the fact that HPV + primary tumours tend to have a more regular shape and some features can correctly assess their rounder appearance. Differently, histogram features study the gray-level distribution frequency within the ROI in order to evaluate the single pixel and not its interactions with adjacent pixels. Always on CT images, Bogowicz et al. [89], Ranjbar et al. [90], Fujita et al. [91] as also Buch at al. [92] assessed how histogram features were able to discriminate HPV status. Differently, de Perrot et al., analysed ADC-MRI sequences [66]. The higher orders assess differences between pixels and voxels in the context of the entire ROI by identifying variations within the examined space in gray-level intensity. Leijenaar at al. [88] evaluated how low-gray-level-large-size-emphasis, was higher in HPV + tumours. Regarding MRI imaging, Dang et al. [98] conducted a study on T1 and T2 weighted images and DWI sequences, with around 80% accuracy in differentiate HPV + from HPV- tumours.
Differential diagnosis
As already said, many studies have tried to evaluate whether radiomics features are able to differentiate malignant and benign lesion or to evaluate HPV status of malignant lesions. Regarding the differentiation of the status, a study by Choi et al. [86] and the one by Yu et al. [87], demonstrate how some features, including a shape one, SphericalDisproportion, was statistically significant in differentiate HPV + from HPV- tumours, due to their less complex shape. Also, histogram features, such as median and entropy, were able to differentiate the HPV status in different studies such as the one by Fujita et al. [91], and the one by Buch et al. [92]. Features representing the tissue heterogeneity were found significant in different studies, with features of heterogeneity with lower values in HPV + tumours, related to their greater structural homogeneity [88, 89].
Radiomics has also been used to try to differentiate OPSCC from other tumours that can affect tissues like tonsils, as for example, lymphoma as in the study by Bae et al. [99] on MRI imaging, due to the grater heterogeneity of OPSCC tumour different from lymphatic tissue.
Texture analysis has been used also to discriminate normal tonsillar tissue from neoplastic one, basing the study not only on structural, morphological and dimensional parameters, but also on the heterogeneity of the structure, as in the study by Kim et al. [100].
Prognostic evaluation and follow-up
The evaluation of prognostic features capable of assessing patients with high risk of distant metastases in HPV + OPSCC is very important in order to establish the correct treatement. In a study by Rich et al. [101] they found out some features able to separate high risk patients from low risks ones. Other important application is the evaluation of neoplasms at a high risk of non-response or recurrence after therapy. This is in particular an important tool, that in the future could be of great aid in the correct therapeutic decision-making, in order to potentially enable personalized radiotherapy treatment [102–104]; regarding this application, the study by M.D. Anderson Cancer Center, Houston, Texas, USA [105] performed on 465 patients, has tried to correlate response to therapy in OPSCC patients, with different clinical prognostic factors. The research of radiomics features capable of creating prognostic and risk model have been applied both in CT and PET/CT imaging [100, 106–109]. One of these, by Cozzi et al. [110]; assessed how some features correlate with survival and local control after RT-CHT.
Limits
Although radiomics is not yet used in clinical practice, it may become in the future a valuable aid in the diagnosis, evaluation of HPV status and therefore aggressiveness of squamous cell tumours and, therefore, in decision-making process. There are currently limitations to the use of texture analysis. One of the challenges is the necessary implementation of standardised methods for the analysis of textural parameters, due to the large number of different procedures and software that can be used (free and non-free commercially available or custom-made-in-house applications) [111–113]. In addition, the great amount of variables during the execution of the examination, both on CT and MRI (different machines used, types of acquisition or sequences, post-processing algorithms, type of software used) also make the results difficult to reproduce at this time [114–116]. Also, the full data processing should be conducted in the shortest possible time with the possibility to analyse them by implementing the software systems where radiologists are used to visualise images. The addition of texture analysis should lead to improved diagnostic performance and in clinical practice, should be integrated with other CT, MRI and clinical parameters to better characterize the tissue investigated [106, 117].
Conclusions
HPV-related cancer of the oropharynx is characterized by different treatment options and prognosis compared to its HPV- counterpart. It is thus of paramount importance to identify means to distinguish between the two entities. While at present biopsy is the gold standard for diagnosis, there are a plethora of imaging criteria that can help the radiologist infer the nature of a primary lesion of the oropharynx or of a metastatic lymph node. Knowledge of these criteria is fundamental for any radiologist that deals with the head and neck district and will become more and more important as HPV-related disease continues to rise in numbers.
Authors’ contributions
Author contributions LC, EB and LB contributed to conceptualization; VG, VM contributed to methodology; FDM and FM contributed to resources; LC, EB and LB, contributed to writing— original draft preparation; FM, LC, EB and RF contributed to writing—review and editing; VG contributed to visualization; LC, LB and FM prepared figures and tables; VM and VG contributed to supervision and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.O’Rorke MA, et al. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191–201. doi: 10.1016/j.oraloncology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Ando T, Kato H, Matsuo M. Different CT imaging findings between histological subtypes in patients with primary thyroid lymphoma. Radiol Med. 2022;127(2):191–8. doi: 10.1007/s11547-022-01447-y. [DOI] [PubMed] [Google Scholar]
- 4.Mungai F, et al. Imaging biomarkers in the diagnosis of salivary gland tumors: the value of lesion/parenchyma ratio of perfusion-MR pharmacokinetic parameters. Radiol Med. 2021;126(10):1345–55. doi: 10.1007/s11547-021-01376-2. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza G, et al. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS ONE. 2014;9(1):e86023. doi: 10.1371/journal.pone.0086023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farsi NJ, et al. Aetiological heterogeneity of head and neck squamous cell carcinomas: the role of human papillomavirus infections, smoking and alcohol. Carcinogenesis. 2017;38(12):1188–95. doi: 10.1093/carcin/bgx106. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka TI, Alawi F. Human papillomavirus and Oropharyngeal Cancer. Dent Clin North Am. 2018;62(1):111–20. doi: 10.1016/j.cden.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Pinkiewicz M, Dorobisz K, Zatonski T. Human Papillomavirus-Associated Head and Neck Cancers. Where are we now? A systematic review. Cancer Manag Res. 2022;14:3313–24. doi: 10.2147/CMAR.S379173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta V, et al. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope. 2013;123(1):146–51. doi: 10.1002/lary.23562. [DOI] [PubMed] [Google Scholar]
- 10.Karpathiou G, et al. p16 and p53 expression status in head and neck squamous cell carcinoma: a correlation with histological, histoprognostic and clinical parameters. Pathology. 2016;48(4):341–8. doi: 10.1016/j.pathol.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Augustin JG, et al. HPV Detection in Head and Neck squamous cell carcinomas: what is the issue? Front Oncol. 2020;10:1751. doi: 10.3389/fonc.2020.01751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantrell SC, et al. Differences in imaging characteristics of HPV-positive and HPV-Negative oropharyngeal cancers: a blinded matched-pair analysis. AJNR Am J Neuroradiol. 2013;34(10):2005–9. doi: 10.3174/ajnr.A3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15(22):6758–62. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 15.Huang YH, et al. Cystic nodal metastasis in patients with oropharyngeal squamous cell carcinoma receiving chemoradiotherapy: relationship with human papillomavirus status and failure patterns. PLoS ONE. 2017;12(7):e0180779. doi: 10.1371/journal.pone.0180779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsch CFE, Schmalfuss IM. Practical Tips for MR Imaging of Perineural Tumor Spread. Magn Reson Imaging Clin N Am. 2018;26(1):85–100. doi: 10.1016/j.mric.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Maraghelli D, et al. Techniques, Tricks, and stratagems of oral cavity computed tomography and magnetic resonance imaging. Appl Sci. 2022;12(3):1473. [Google Scholar]
- 18.Petralia G, et al. Dynamic contrast-enhanced MRI in oncology: how we do it. Radiol Med. 2020;125(12):1288–300. doi: 10.1007/s11547-020-01220-z. [DOI] [PubMed] [Google Scholar]
- 19.Iacobellis F, et al. Role of MRI in early follow-up of patients with solid organ injuries: how and why we do it? Radiol Med. 2021;126(10):1328–34. doi: 10.1007/s11547-021-01394-0. [DOI] [PubMed] [Google Scholar]
- 20.Fakhry C, et al. The use of ultrasound in the search for the primary site of unknown primary head and neck squamous cell cancers. Oral Oncol. 2014;50(7):640–5. doi: 10.1016/j.oraloncology.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahuja A, et al. A practical approach to ultrasound of cervical lymph nodes. J Laryngol Otol. 1997;111(3):245–56. doi: 10.1017/s0022215100137004. [DOI] [PubMed] [Google Scholar]
- 22.Ahuja A, Ying M. Sonography of neck lymph nodes. Part II: abnormal lymph nodes. Clin Radiol. 2003;58(5):359–66. doi: 10.1016/s0009-9260(02)00585-8. [DOI] [PubMed] [Google Scholar]
- 23.Qin H, et al. Magnetic resonance imaging (MRI) radiomics of papillary thyroid cancer (PTC): a comparison of predictive performance of multiple classifiers modeling to identify cervical lymph node metastases before surgery. Radiol Med. 2021;126(10):1312–27. doi: 10.1007/s11547-021-01393-1. [DOI] [PubMed] [Google Scholar]
- 24.Bicci E et al. Role of texture analysis in Oropharyngeal Carcinoma: a systematic review of the literature. Cancers (Basel), 2022. 14(10). [DOI] [PMC free article] [PubMed]
- 25.Kawaguchi M, et al. Comparison of imaging findings between human papillomavirus-positive and -negative squamous cell carcinomas of the Maxillary Sinus. J Clin Imaging Sci. 2020;10:59. doi: 10.25259/JCIS_116_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao X, et al. Performance of different imaging techniques in the diagnosis of head and neck cancer mandibular invasion: a systematic review and meta-analysis. Oral Oncol. 2018;86:150–64. doi: 10.1016/j.oraloncology.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 27.van den Brekel MW, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990;177(2):379–84. doi: 10.1148/radiology.177.2.2217772. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, et al. Sonographic assessment of cervical lymphadenopathy: role of high-resolution and color doppler imaging. Head Neck. 2011;33(3):297–302. doi: 10.1002/hed.21448. [DOI] [PubMed] [Google Scholar]
- 29.Chan MW, et al. Morphologic and topographic radiologic features of human papillomavirus-related and -unrelated oropharyngeal carcinoma. Head Neck. 2017;39(8):1524–34. doi: 10.1002/hed.24764. [DOI] [PubMed] [Google Scholar]
- 30.Salzillo TC, et al. Advances in imaging for HPV-Related Oropharyngeal Cancer: applications to Radiation Oncology. Semin Radiat Oncol. 2021;31(4):371–88. doi: 10.1016/j.semradonc.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato MG, et al. Update on oral and oropharyngeal cancer staging - international perspectives. World J Otorhinolaryngol Head Neck Surg. 2020;6(1):66–75. doi: 10.1016/j.wjorl.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvestrini V, et al. The impact of patient preference in the treatment algorithm for recurrent/metastatic head and neck squamous cell carcinoma. Radiol Med. 2022;127(8):866–71. doi: 10.1007/s11547-022-01509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldenberg D, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 34.Yasui T, et al. Human papillomavirus and cystic node metastasis in oropharyngeal cancer and cancer of unknown primary origin. PLoS ONE. 2014;9(4):e95364. doi: 10.1371/journal.pone.0095364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kane SV, et al. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur J Surg Oncol. 2006;32(7):795–803. doi: 10.1016/j.ejso.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Hubert Low TH, et al. Tumor classification for early oral cancer: re-evaluate the current TNM classification. Head Neck. 2015;37(2):223–8. doi: 10.1002/hed.23581. [DOI] [PubMed] [Google Scholar]
- 37.International Consortium for Outcome Research in Primary tumor staging for oral cancer and a proposed modification incorporating depth of invasion: an international multicenter retrospective study. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1138–48. doi: 10.1001/jamaoto.2014.1548. [DOI] [PubMed] [Google Scholar]
- 38.Byers RM, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck. 1998;20(2):138–44. doi: 10.1002/(sici)1097-0347(199803)20:2<138::aid-hed7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Wippold FJ 2nd. Head and neck imaging: the role of CT and MRI. J Magn Reson Imaging. 2007;25(3):453–65. [DOI] [PubMed]
- 40.van Dijke CF, van Waes PF. Head and neck tumors, MRI versus CT: a technology assessment pilot study. Eur J Radiol. 1992;14(3):235–9. doi: 10.1016/0720-048x(92)90094-p. [DOI] [PubMed] [Google Scholar]
- 41.Avery EW et al. Role of PET/CT in Oropharyngeal Cancers. Cancers (Basel), 2023. 15(9). [DOI] [PMC free article] [PubMed]
- 42.Lowe VJ, et al. Multicenter Trial of [(18)F]fluorodeoxyglucose Positron Emission Tomography/Computed tomography staging of Head and Neck Cancer and negative Predictive Value and Surgical Impact in the N0 Neck: results from ACRIN 6685. J Clin Oncol. 2019;37(20):1704–12. doi: 10.1200/JCO.18.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noij DP, et al. Diagnostic value of diffusion-weighted imaging and (18)F-FDG-PET/CT for the detection of unknown primary head and neck cancer in patients presenting with cervical metastasis. Eur J Radiol. 2018;107:20–5. doi: 10.1016/j.ejrad.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Chen B, et al. Diagnostic performance of 18F-FDG PET/CT for the detection of occult primary tumors in squamous cell carcinoma of unknown primary in the head and neck: a single-center retrospective study. Nucl Med Commun. 2021;42(5):523–7. doi: 10.1097/MNM.0000000000001365. [DOI] [PubMed] [Google Scholar]
- 45.Touska P, Connor S. Imaging of human papilloma virus associated oropharyngeal squamous cell carcinoma and its impact on diagnosis, prognostication, and response assessment. Br J Radiol. 2022;95(1138):20220149. doi: 10.1259/bjr.20220149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Shwaiheen FA, et al. The advantages and drawbacks of routine magnetic resonance imaging for long-term post-treatment locoregional surveillance of oral cavity squamous cell carcinoma. Am J Otolaryngol. 2015;36(3):415–23. doi: 10.1016/j.amjoto.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 47.Mes SW, et al. Outcome prediction of head and neck squamous cell carcinoma by MRI radiomic signatures. Eur Radiol. 2020;30(11):6311–21. doi: 10.1007/s00330-020-06962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nooij RP, et al. Functional MRI for treatment evaluation in patients with Head and Neck squamous cell carcinoma: a review of the literature from a Radiologist Perspective. Curr Radiol Rep. 2018;6(1):2. doi: 10.1007/s40134-018-0262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boland PW, et al. A detailed anatomical assessment of the lateral tongue extrinsic musculature, and proximity to the tongue mucosal surface. Does this confirm the current TNM T4a muscular subclassification? Surg Radiol Anat. 2013;35(7):559–64. doi: 10.1007/s00276-013-1076-6. [DOI] [PubMed] [Google Scholar]
- 50.Burke CJ, Thomas RH, Howlett D. Imaging the major salivary glands. Br J Oral Maxillofac Surg. 2011;49(4):261–9. doi: 10.1016/j.bjoms.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Maraghelli D, et al. Magnetic resonance imaging of salivary gland tumours: key findings for imaging characterisation. Eur J Radiol. 2021;139:109716. doi: 10.1016/j.ejrad.2021.109716. [DOI] [PubMed] [Google Scholar]
- 52.Lo Casto A, et al. Diagnostic and prognostic value of magnetic resonance imaging in the detection of tumor depth of invasion and bone invasion in patients with oral cavity cancer. Radiol Med. 2022;127(12):1364–72. doi: 10.1007/s11547-022-01565-7. [DOI] [PubMed] [Google Scholar]
- 53.Kreimer AR, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 54.Piludu F et al. Multiparametric MRI evaluation of Oropharyngeal squamous cell carcinoma. A mono-institutional study. J Clin Med, 2021. 10(17). [DOI] [PMC free article] [PubMed]
- 55.Ravanelli M, et al. Correlation between human papillomavirus status and quantitative MR Imaging Parameters including diffusion-weighted imaging and texture features in Oropharyngeal Carcinoma. AJNR Am J Neuroradiol. 2018;39(10):1878–83. doi: 10.3174/ajnr.A5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Assadsangabi R, et al. Multimodality oncologic evaluation of superficial neck and facial lymph nodes. Radiol Med. 2021;126(8):1074–84. doi: 10.1007/s11547-021-01367-3. [DOI] [PubMed] [Google Scholar]
- 57.Ailianou A, et al. MRI with DWI for the detection of Posttreatment Head and Neck squamous cell carcinoma: why morphologic MRI Criteria Matter. AJNR Am J Neuroradiol. 2018;39(4):748–55. doi: 10.3174/ajnr.A5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chawla S, et al. Pretreatment diffusion-weighted and dynamic contrast-enhanced MRI for prediction of local treatment response in squamous cell carcinomas of the head and neck. AJR Am J Roentgenol. 2013;200(1):35–43. doi: 10.2214/AJR.12.9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uribe S, Rojas LA, Rosas CF. Accuracy of imaging methods for detection of bone tissue invasion in patients with oral squamous cell carcinoma. Dentomaxillofac Radiol. 2013;42(6):20120346. doi: 10.1259/dmfr.20120346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan WJ, et al. Diffusion-weighted imaging as a follow-up modality for evaluation of major salivary gland function in nasopharyngeal carcinoma patients: a preliminary study. Strahlenther Onkol. 2020;196(6):530–41. doi: 10.1007/s00066-020-01580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khoo MM, et al. Diffusion-weighted imaging (DWI) in musculoskeletal MRI: a critical review. Skeletal Radiol. 2011;40(6):665–81. doi: 10.1007/s00256-011-1106-6. [DOI] [PubMed] [Google Scholar]
- 62.Shi D, et al. Salivary gland function in nasopharyngeal carcinoma before and late after intensity-modulated radiotherapy evaluated by dynamic diffusion-weighted MR imaging with gustatory stimulation. BMC Oral Health. 2019;19(1):288. doi: 10.1186/s12903-019-0951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Widmann G, et al. MRI sequences in Head & Neck Radiology - State of the art. Rofo. 2017;189(5):413–22. doi: 10.1055/s-0043-103280. [DOI] [PubMed] [Google Scholar]
- 64.Juan CJ, et al. Perfusion characteristics of late radiation injury of parotid glands: quantitative evaluation with dynamic contrast-enhanced MRI. Eur Radiol. 2009;19(1):94–102. doi: 10.1007/s00330-008-1104-9. [DOI] [PubMed] [Google Scholar]
- 65.Patel P, et al. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol. 2017;19(1):118–27. doi: 10.1093/neuonc/now148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Perrot T, et al. Apparent diffusion coefficient histograms of human papillomavirus-positive and human papillomavirus-negative Head and Neck squamous cell carcinoma: Assessment of Tumor Heterogeneity and comparison with histopathology. AJNR Am J Neuroradiol. 2017;38(11):2153–60. doi: 10.3174/ajnr.A5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lenoir V, et al. Diffusion-weighted imaging to assess HPV-Positive versus HPV-Negative Oropharyngeal squamous cell carcinoma: the importance of b-Values. AJNR Am J Neuroradiol. 2022;43(6):905–12. doi: 10.3174/ajnr.A7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jansen JF, et al. Correlation of a priori DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: initial analysis. Oral Oncol. 2012;48(8):717–22. doi: 10.1016/j.oraloncology.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chawla S, et al. Prediction of distant metastases in patients with squamous cell carcinoma of head and neck using DWI and DCE-MRI. Head Neck. 2020;42(11):3295–306. doi: 10.1002/hed.26386. [DOI] [PubMed] [Google Scholar]
- 70.Han M et al. Correlation of human papilloma virus status with quantitative perfusion/diffusion/metabolic imaging parameters in the oral cavity and oropharyngeal squamous cell carcinoma: comparison of primary tumour sites and metastatic lymph nodes. Clin Radiol, 2018. 73(8): p. 757 e21-757 e27. [DOI] [PubMed]
- 71.Giannitto C, et al. An approach to evaluate the quality of radiological reports in Head and Neck cancer loco-regional staging: experience of two academic hospitals. Radiol Med. 2022;127(4):407–13. doi: 10.1007/s11547-022-01464-x. [DOI] [PubMed] [Google Scholar]
- 72.Nardi C, et al. Texture analysis in the characterization of parotid salivary gland lesions: a study on MR diffusion weighted imaging. Eur J Radiol. 2021;136:109529. doi: 10.1016/j.ejrad.2021.109529. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L, et al. Computed tomography-based radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors. Radiol Med. 2020;125(5):465–73. doi: 10.1007/s11547-020-01138-6. [DOI] [PubMed] [Google Scholar]
- 74.Kirienko M, et al. Computed tomography (CT)-derived radiomic features differentiate prevascular mediastinum masses as thymic neoplasms versus lymphomas. Radiol Med. 2020;125(10):951–60. doi: 10.1007/s11547-020-01188-w. [DOI] [PubMed] [Google Scholar]
- 75.Santone A, et al. Radiomic features for prostate cancer grade detection through formal verification. Radiol Med. 2021;126(5):688–97. doi: 10.1007/s11547-020-01314-8. [DOI] [PubMed] [Google Scholar]
- 76.Karmazanovsky G et al. Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol Med, 2021. [DOI] [PubMed]
- 77.Nardone V, et al. Delta radiomics: a systematic review. Radiol Med. 2021;126(12):1571–83. doi: 10.1007/s11547-021-01436-7. [DOI] [PubMed] [Google Scholar]
- 78.Palatresi D, et al. Correlation of CT radiomic features for GISTs with pathological classification and molecular subtypes: preliminary and monocentric experience. Radiol Med. 2022;127(2):117–28. doi: 10.1007/s11547-021-01446-5. [DOI] [PubMed] [Google Scholar]
- 79.Granata V et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol Med, 2022. [DOI] [PubMed]
- 80.Lubner MG, et al. CT texture analysis: definitions, applications, Biologic correlates, and Challenges. Radiographics. 2017;37(5):1483–503. doi: 10.1148/rg.2017170056. [DOI] [PubMed] [Google Scholar]
- 81.Scapicchio C, et al. A deep look into radiomics. Radiol Med. 2021;126(10):1296–311. doi: 10.1007/s11547-021-01389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coppola F, et al. Artificial intelligence: radiologists’ expectations and opinions gleaned from a nationwide online survey. Radiol Med. 2021;126(1):63–71. doi: 10.1007/s11547-020-01205-y. [DOI] [PubMed] [Google Scholar]
- 83.Benedetti G, et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol Med. 2021;126(6):745–60. doi: 10.1007/s11547-021-01333-z. [DOI] [PubMed] [Google Scholar]
- 84.Satake H, et al. Radiomics in breast MRI: current progress toward clinical application in the era of artificial intelligence. Radiol Med. 2022;127(1):39–56. doi: 10.1007/s11547-021-01423-y. [DOI] [PubMed] [Google Scholar]
- 85.Autorino R et al. Radiomics-based prediction of two-year clinical outcome in locally advanced cervical cancer patients undergoing neoadjuvant chemoradiotherapy. Radiol Med, 2022. [DOI] [PMC free article] [PubMed]
- 86.Choi Y, et al. Prediction of human papillomavirus status and overall survival in patients with untreated oropharyngeal squamous cell carcinoma: development and validation of CT-Based Radiomics. AJNR Am J Neuroradiol. 2020;41(10):1897–904. doi: 10.3174/ajnr.A6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu K, et al. Radiomic analysis in prediction of human papilloma virus status. Clin Transl Radiat Oncol. 2017;7:49–54. doi: 10.1016/j.ctro.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leijenaar RT, et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol. 2018;91(1086):20170498. doi: 10.1259/bjr.20170498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bogowicz M, et al. Computed Tomography Radiomics predicts HPV Status and local Tumor Control after definitive Radiochemotherapy in Head and Neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2017;99(4):921–8. doi: 10.1016/j.ijrobp.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 90.Ranjbar S, et al. Computed tomography-based texture analysis to Determine Human Papillomavirus Status of Oropharyngeal squamous cell carcinoma. J Comput Assist Tomogr. 2018;42(2):299–305. doi: 10.1097/RCT.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 91.Fujita A, et al. Difference between HPV-Positive and HPV-Negative non-oropharyngeal head and Neck Cancer: texture analysis features on CT. J Comput Assist Tomogr. 2016;40(1):43–7. doi: 10.1097/RCT.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 92.Buch K, et al. Using texture analysis to Determine Human Papillomavirus Status of Oropharyngeal squamous cell carcinomas on CT. AJNR Am J Neuroradiol. 2015;36(7):1343–8. doi: 10.3174/ajnr.A4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fung N, et al. The role of human papillomavirus on the prognosis and treatment of oropharyngeal carcinoma. Cancer Metastasis Rev. 2017;36(3):449–61. doi: 10.1007/s10555-017-9686-9. [DOI] [PubMed] [Google Scholar]
- 94.Dahlstrom KR, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119(1):81–9. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maddalo M, et al. A pilot study on the Vanderbilt head and neck symptom survey italian version (VHNSS-IT) to test its feasibility and utility in routine clinical practice. Radiol Med. 2020;125(4):423–31. doi: 10.1007/s11547-019-01125-6. [DOI] [PubMed] [Google Scholar]
- 96.Maddalo M, et al. The linguistic validation process of the Vanderbilt Head and Neck Symptom Survey - Italian Version (VHNSS-IT) Radiol Med. 2020;125(2):228–35. doi: 10.1007/s11547-019-01105-w. [DOI] [PubMed] [Google Scholar]
- 97.Bos P, et al. Clinical variables and magnetic resonance imaging-based radiomics predict human papillomavirus status of oropharyngeal cancer. Head Neck. 2021;43(2):485–95. doi: 10.1002/hed.26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dang M, et al. MRI texture analysis predicts p53 status in head and neck squamous cell carcinoma. AJNR Am J Neuroradiol. 2015;36(1):166–70. doi: 10.3174/ajnr.A4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bae S, et al. Squamous cell carcinoma and lymphoma of the Oropharynx: differentiation using a Radiomics Approach. Yonsei Med J. 2020;61(10):895–900. doi: 10.3349/ymj.2020.61.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim TY, et al. CT texture analysis of tonsil cancer: discrimination from normal palatine tonsils. PLoS ONE. 2021;16(8):e0255835. doi: 10.1371/journal.pone.0255835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rich B et al. Radiomics Predicts for Distant Metastasis in Locally Advanced Human Papillomavirus-Positive Oropharyngeal Squamous Cell Carcinoma. Cancers (Basel), 2021. 13(22). [DOI] [PMC free article] [PubMed]
- 102.De Felice F, et al. A snapshot on radiotherapy for head and neck cancer patients during the COVID-19 pandemic: a survey of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) head and neck working group. Radiol Med. 2021;126(2):343–7. doi: 10.1007/s11547-020-01296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Felice F, Musio D, Tombolini V. Weekly hypofractionated radiation therapy in elderly non-resectable cutaneous squamous cell carcinoma of the head and neck region. Radiol Med. 2021;126(4):620–2. doi: 10.1007/s11547-020-01260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merlotti A, et al. Sequential chemo-hypofractionated RT versus concurrent standard CRT for locally advanced NSCLC: GRADE recommendation by the Italian Association of Radiotherapy and Clinical Oncology (AIRO) Radiol Med. 2021;126(8):1117–28. doi: 10.1007/s11547-021-01362-8. [DOI] [PubMed] [Google Scholar]
- 105.Head MDACC. Neck quantitative imaging Working, Investigation of radiomic signatures for local recurrence using primary tumor texture analysis in oropharyngeal head and neck cancer patients. Sci Rep. 2018;8(1):1524. doi: 10.1038/s41598-017-14687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller TA, et al. Prognostic value of pre-treatment CT texture analysis in combination with change in size of the primary tumor in response to induction chemotherapy for HPV-positive oropharyngeal squamous cell carcinoma. Quant Imaging Med Surg. 2019;9(3):399–408. doi: 10.21037/qims.2019.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuno H, et al. Texture analysis potentially predicts local failure in Head and Neck squamous cell carcinoma treated with Chemoradiotherapy. AJNR Am J Neuroradiol. 2017;38(12):2334–40. doi: 10.3174/ajnr.A5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haider SP, et al. PET/CT radiomics signature of human papilloma virus association in oropharyngeal squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2020;47(13):2978–91. doi: 10.1007/s00259-020-04839-2. [DOI] [PubMed] [Google Scholar]
- 109.Haider SP et al. Potential added value of PET/CT Radiomics for Survival Prognostication beyond AJCC 8th Edition staging in Oropharyngeal squamous cell carcinoma. Cancers (Basel), 2020. 12(7). [DOI] [PMC free article] [PubMed]
- 110.Cozzi L, et al. Predicting survival and local control after radiochemotherapy in locally advanced head and neck cancer by means of computed tomography based radiomics. Strahlenther Onkol. 2019;195(9):805–18. doi: 10.1007/s00066-019-01483-0. [DOI] [PubMed] [Google Scholar]
- 111.Fujima N, et al. The utility of MRI histogram and texture analysis for the prediction of histological diagnosis in head and neck malignancies. Cancer Imaging. 2019;19(1):5. doi: 10.1186/s40644-019-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bicci E, Nardi C, Calamandrei L, Barcali E, Pietragalla M, Calistri L, Desideri I, Mungai F, Bonasera L, Miele V. Magnetic resonance imaging in naso-oropharyngeal carcinoma: role of texture analysis in the assessment of response to radiochemotherapy, a preliminary study. Radiol Med. 2023;128(7):839–52. doi: 10.1007/s11547-023-01653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wahid KA, et al. Intensity standardization methods in magnetic resonance imaging of head and neck cmillerancer. Phys Imaging Radiat Oncol. 2021;20:88–93. doi: 10.1016/j.phro.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Davnall F, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3(6):573–89. doi: 10.1007/s13244-012-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bicci E, Cozzi D, Cavigli E, Ruzga R, Bertelli E, Danti G, Bettarini S, Tortoli P, Mazzoni LN, Busoni S, Miele V. Reproducibility of CT radiomic features in lung neuroendocrine tumours (NETs) patients: analysis in a heterogeneous population. Radiol Med. 2023;128(2):203–11. doi: 10.1007/s11547-023-01592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mackin D, et al. Measuring computed tomography scanner variability of radiomics features. Invest Radiol. 2015;50(11):757–65. doi: 10.1097/RLI.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Calamandrei L, et al. Morphological, functional and texture analysis magnetic resonance imaging features in the assessment of radiotherapy-induced xerostomia in oropharyngeal cancer. Appl Sci. 2023;13(2):810. doi: 10.3390/app13020810. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.