Abstract
Introduction:
In hospitalized patients, continuous glucose monitoring (CGM) may improve glycemic control, prevent hypoglycemic events, and reduce staff workload compared with point-of-care (POC) capillary glucose monitoring.
Methods:
To evaluate CGM accuracy and safety of use in the inpatient setting, two versions of CGM sensors were placed on 43 and 34 adult patients with diabetes admitted to non-intensive care unit (ICU) medical wards, respectively. CGM accuracy relative to POC and safety of use were measured by calculating mean absolute relative difference (MARD) and by Clarke Error Grid (CEG) analysis.
Results:
CGM version 2 had improved accuracy compared with CGM version 1 with MARD 17.7 compared with 21.4%. CGM accuracy did not change with POC value or with time of sensor wear. On CEG, 98.8% of paired values fell within acceptable zones A and B.
Conclusion:
Despite reduced accuracy compared with the outpatient setting, both versions of CGMs had acceptable safety profiles in the inpatient setting.
Keywords: continuous glucose monitor (CGM), diabetes technology, inpatient diabetes management, type 1 diabetes (T1DM), type 2 diabetes (T2DM)
Introduction
Maintenance of glycemic control in hospitalized patients is generally achieved by monitoring point-of-care (POC) capillary glucose levels to guide adjustments in treatment. This process involves frequent nurse-patient interaction several times per day. This has been driven by the well-established data that glycemic control improves patient outcomes. 1 Continuous glucose monitoring (CGM) devices have been widely used in the outpatient setting and can improve glycemic control. 2 However, data on the accuracy of these devices in the hospital setting are limited. 3 There has been increased interest in using these devices in the hospital setting from providers and patients because of their convenience, ability to monitor glucose more frequently and observe glucose trends, and opportunities for remote monitoring. This has been amplified by the coronavirus disease of 2019 (COVID-19) pandemic and the need to limit exposure of health care providers and conserve personal protective equipment. 4
The two factory-calibrated CGMs currently in wide use in the United States are made by Dexcom (San Diego, CA) and by Abbott (Alameda, CA). Three small studies have shown reasonable accuracy using the Dexcom system in the inpatient setting,4-6 and one recent study evaluated the patient-blinded Abbott Freestyle Libre Pro system in hospitalized patients. 7 To our knowledge, no studies on the inpatient use of the recently updated Freestyle Libre 2 CGM have been published. We report the first study evaluating the safety and accuracy of the 14-day Freestyle Libre (CGM-1) and Libre 2 CGMs (CGM-2) in the hospital setting.
Methods
Participant Recruitment and CGM Management
Nonpregnant adults with diabetes mellitus requiring insulin therapy who were admitted to non-intensive care unit (ICU) medical wards were included in the study. Those undergoing recent or anticipated surgery, requiring dialysis, taking hydroxyurea, high-dose acetaminophen or vitamin C, and those with significant pitting edema or significantly altered mental status were excluded. After informed consent, a CGM sensor was placed on subjects’ upper arms. Sensor version was assigned based on sensor availability. Participants were provided with CGM readers or had the LibreLink App installed on their compatible smartphone and were educated on how to scan the sensor. Subjects were encouraged to scan the sensor with at least every POC measurement. Use of glucose alerts available with CGM-2 was optional and was not part of this study. CGM data were uploaded securely to the LibreView online server for subsequent analysis. Subjects received standard-of-care insulin dosing and POC capillary glucose monitoring using the US Food and Drug Administration (FDA)-approved Accu-Chek Inform II system. The study was approved by the Vanderbilt University Medical Center Institutional Review Board.
Data Analysis
POC capillary glucose values and clinical data, including age, sex, weight, diabetes type, hemoglobin A1c (HbA1c), and creatinine on admission, were obtained from the electronic health record. CGM values were paired with the POC value in closest temporal proximity, excluding values that did not have a pair within 15 minutes. Relative differences (RD) were calculated as RD = 100 * (xCGM—xPOC)/xPOC. MARD, the mean of |RD|, was calculated for individual participants and for the group. Linear regression analysis was performed to evaluate correlation between RD and POC glucose or sensor time of wear. Multiple regression analysis was performed to evaluate for correlation between individual MARDs and age, sex, body weight, HbA1c, and renal function. Clarke Error Grid (CEG) analysis was performed as previously described. 8 All calculations were performed using Microsoft Excel and analyses were performed using Graphpad Prism software.
Results
Patient Characteristics
Median participant age was 57.5 years (interquartile range [IQR] = 47.9-64.9). Thirty-two of 77 subjects were female, and 60 were characterized as having type 2 diabetes, 13 as type 1 diabetes, and 4 as unknown type. Median weight was 99.8 kg (IQR = 87.0-111.3). Median admission creatinine was 1.25 mg/dL (IQR = 0.96-1.87) and median HbA1c was 8.7% (IQR = 7.2-11.1). Median length of CGM use was two days, with a range of one to nine days. Four participants were excluded due to failure to scan the CGM during their hospital admission, and one was excluded due to erratic and unreliable blood glucose readings on both CGM and POC testing.
Inpatient CGM Accuracy and Safety
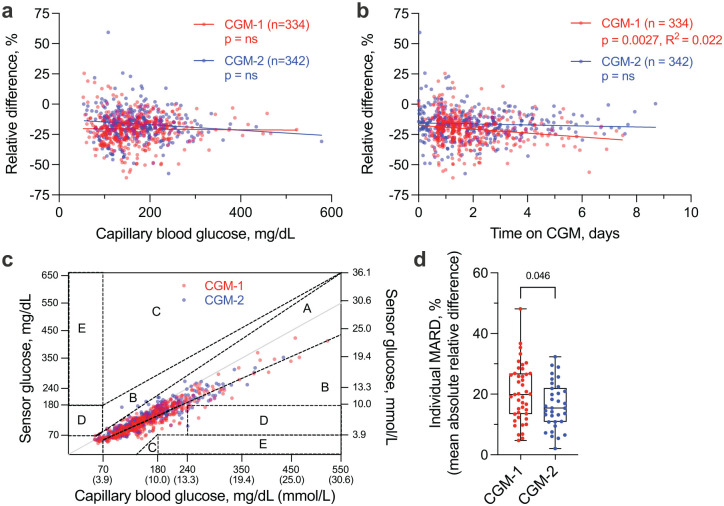
CGM-2 accuracy relative to POC was slightly improved compared with CGM-1 (MARD 17.7 ± 10.5 vs 21.4 ± 11.8%, P < .0001), with MARDs fairly consistent across the glycemic range (Table 1). Hypoglycemia was rare in both groups of patients. For both systems, CGM values were consistently lower than POC values, and their RDs persisted regardless of POC value (Figure 1a). CGM-1 had a minimal decrease in accuracy over time, whereas CGM-2 had no change in accuracy with time (Figure 1b). On CEG analysis, 98.8% of all values fell within the optimal (zone A) or acceptable (zone B) regions (Figure 1c), showing minimal risk for inappropriate treatments of hypo- or hyperglycemia. MARD values varied markedly between individuals in both CGM-1 and CGM-2 groups (Figure 1d). We identified no correlation between individual MARD and patient age, sex, weight, type of diabetes, admission HbA1c, or admission creatinine level on multiple regression analysis.
Table 1.
Mean Absolute Relative Differences of CGM-1 and CGM-2 Compared With Capillary Blood Glucose.
| Capillary blood glucose, mg/dL | CGM-1 MARD, % (n) | CGM-2 MARD, % (n) |
|---|---|---|
| Overall | 21.4 (334) | 17.7 (342) |
| <70 | 16.2 (12) | 7.6 (5) |
| 70-180 | 22.5 (202) | 17.7 (204) |
| 180-250 | 20.8 (83) | 17.8 (99) |
| >250 | 19.0 (37) | 19.0 (34) |
Abbreviations: CGM, continuous glucose monitoring; MARD, mean absolute relative difference.
Figure 1.
CGM-1 and CGM-2 accuracy in adult hospitalized patients. (a-b) CGM relative difference compared with POC capillary blood glucose remains unchanged regardless of POC value and length of time on sensor. Points represent individual paired CGM and POC values. Line represents slope of the mean of individual values. (c) 98.8% of values fall in acceptable zones A and B of the Clarke Error Grid. (d) MARD variability between individual participants is high; points represent individual participants. n = 43 (CGM-1) and 34 (CGM-2) participants with a total of 676 paired glucose values. Abbreviations: CGM, continuous glucose monitoring; POC, point-of-care; MARD, mean absolute relative difference.
Discussion
CGM has great potential to improve patient care and nursing safety in the hospital, but the safety and accuracy of currently available CGM systems is not yet well-established. To our knowledge, this study provides the first assessment of the safety and accuracy of CGM-2 for inpatient use. In a recent study comparing the performance of the Freestyle Libre CGM and POC capillary glucose testing in hospitalized patients, Galindo et al 7 demonstrated improved detection of hypoglycemia, partially accounted for by a tendency for the Libre to report lower glucose levels compared with POC testing. Our study in part replicates this finding with CGM glucose values being consistently lower than paired POC values. This discrepancy in CGM and POC values may serve to improve patient safety by increasing detection of hypoglycemic events, though a low overall rate of hypoglycemia in this study precluded direct comparison of the abilities of CGM and POC to detect hypoglycemia. Future studies are needed to clarify how CGM use influences treatment of hypoglycemia in hospitalized patients. CGM-1 and CGM-2 are slightly less accurate overall in our study than reported by Galindo et al. We think this discrepancy is likely due to a broader time allowance for paired values (15 minutes in our study, compared with 5 minutes by Galindo et al). Our participants also wore the sensor for a shorter length of time on average. Notably, CGM-2 was found to have slightly better overall accuracy and no loss in accuracy with duration of sensor wear compared with the CGM-1. Clinical variables including age, sex, body weight, HbA1c, and renal function were not found to predict CGM accuracy in individuals. We did not identify differences in accuracy between subjects with type 2 diabetes as compared with type 1 diabetes. CEG analysis suggested that inpatient use of both CGM models would likely be safe, with nearly all readings falling in the “acceptable” zones. Similarly high rates of clinical accuracy on CEG analysis have been reported in prior CGM studies.6,7
Our study is limited by the relatively small number of subjects, particularly those who wore sensors longer than three days and those with type 1 diabetes. Our use of POC capillary glucose as comparator, rather than the gold-standard Yellow Springs Instrument, and a relatively short wear-time on many patients likely introduced some degree of variability that contributed to the increased MARDs we observed compared with the reported values in outpatient settings. Considering the known delay in change between plasma and interstitial glucose levels, the time allowed between paired CGM and POC values is also likely to have contributed to variability. Future studies with larger sample sizes and direct comparison with the gold-standard plasma glucose measurement will help to determine the clinical benefits to patients and appropriateness of broader use of CGM systems for hospitalized patients. Dedicated implementation studies will be beneficial in elucidating impact on nursing practice and systems of inpatient care delivery.
Conclusion
This observational study comparing the accuracy of two versions of CGM with routine POC capillary glucose measurements adds to the growing body of literature supporting the safety of use of CGM in the inpatient setting. CEG analysis supports the safety of inpatient use of CGM-1 and CGM-2 despite the finding of slightly lower accuracy of the CGM-1 as compared with prior studies. Our study highlights the need for larger observational and implementation studies aimed at evaluating impacts on patient safety and nursing care.
Footnotes
Abbreviations: HbA1c, hemoglobin A1c; CEG, Clarke Error Grid; CGM, continuous glucose monitor/monitoring; COVID-19, coronavirus disease of 2019; IQR, interquartile range; MARD, mean absolute relative difference; POC, point-of-care; RD, relative difference.
Authorship: All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions: JJW, JMW, MF, and SB designed the study. RGW, EH, SB, and AJW assisted with patient recruitment, sensor placement, and data acquisition. JJW, JMW, SBF, SB, and AJW analyzed and compiled the data. JJW, SBF, and AJW wrote the manuscript, and all authors reviewed and edited the manuscript.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SB has received research funding, paid to her institution, from Dexcom, Novo Nordisk, Mylan, AstraZeneca, and Bristol Myers Squibb. JJW, AJW, SBF, RGW, EH, MF, and JMW report no relevant conflicts of interest. CGM sensors and receivers were provided without restriction by Abbott.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was performed using funding provided by the National Institute of Diabetes and Digestive and Kidney Diseases [DK20593, T32DK7061] and the Doris Duke Charitable Foundation.
Compliance With Ethics Guidelines: This study received approval from the Vanderbilt University Medical Center Institutional Review Board, IRB Study #200879 and was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All subjects provided informed consent to participate in the study.
Data Availability: The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Prior Presentation: This study was presented in part as a poster at the virtual 81st American Diabetes Association Scientific Sessions, June 25–29, 2021.
ORCID iD: Sally B. Friedman  https://orcid.org/0000-0002-2921-9642
https://orcid.org/0000-0002-2921-9642
References
- 1. American Diabetes Association. Diabetes care in the hospital. Sec. 13 in standards of medical care in diabetes—2016. Diabetes Care. 2016;39(suppl 1):S99-S104. [DOI] [PubMed] [Google Scholar]
- 2. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis GM, Galindo RJ, Migdal AL, Umpierrez GE. Diabetes technology in the inpatient setting for management of hyperglycemia. Endocrinol Metab Clin North Am. 2020;49(1):79-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nair BG, Dellinger EP, Flum DR, Rooke GA, Hirsch IB. A pilot study of the feasibility and accuracy of inpatient continuous glucose monitoring. Diabetes Care. 2020;43(11):e168-e169. [DOI] [PubMed] [Google Scholar]
- 5. Spanakis EK, Levitt DL, Siddiqui T, et al. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: the glucose telemetry system. J Diabetes Sci Technol. 2018;12(1):20-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reutrakul S, Genco M, Salinas H, et al. Feasibility of inpatient continuous glucose monitoring during the COVID-19 pandemic: early experience. Diabetes Care. 2020;43(10):e137-e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the FreeStyle Libre Pro flash continuous glucose monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43(11):2730-2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622-628. [DOI] [PubMed] [Google Scholar]