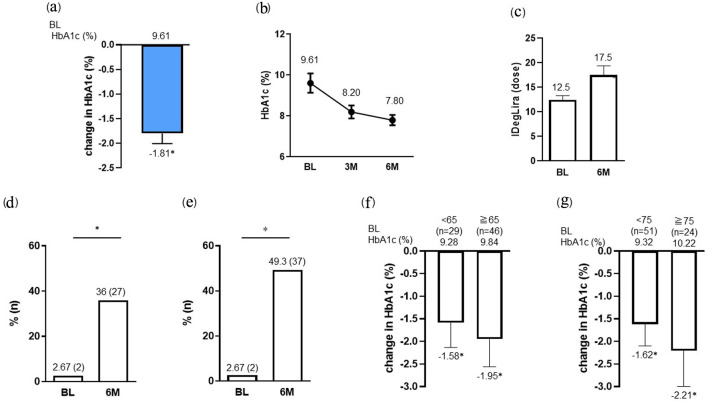
Figure 1.
Change in HbA1c (a, b) and IDegLira dose (c) at 6 months after the initiation of IDegLira. Change in the proportion of patients achieving HbA1c < 7% (d) and the target HbA1c set according to the individual patient’s background (e) at 6 months after the initiation of IDegLira. Change in HbA1c from baseline by subgroups separated by age under 65 or over (f) and under 75 or over (g) years. Data are presented as mean with 95% CIs. *P < 0.01 by Wilcoxon signed-rank sum test (vs. BL HbA1c or dose of IDegLira in a, c, f and g). *P < 0.01 by McNemar’s test (in d and e) (vs. BL). HbA1c: glycated hemoglobin; IDegLira: insulin degludec/liraglutide; CIs: confidence intervals; BL: baseline.