Introduction
In 1980, Jurgen Ludwig, a pathologist at the Mayo Clinic, and colleagues formally described a disorder that involved the accumulation of fat in the liver, independent of significant alcohol consumption, or other secondary causes of chronic hepatic disease.[1] This condition – termed non-alcoholic fatty liver disease (NAFLD) – was specifically defined non-alcoholic to differentiate it from alcoholic fatty liver disease, which exhibits similar histological alterations in the liver but is primarily caused by excessive alcohol consumption. Furthermore, the terminology of non-alcoholic steatohepatitis (NASH) was introduced to characterize the occurrence of inflammatory activity and hepatocyte injury in a steatotic liver tissue, signifying a more progressive stage of the disease.[1] In the spectrum of NAFLD, NASH stands apart from non-alcoholic fatty liver (NAFL), which is typically regarded as a non-progressive condition characterized by macrovesicular steatosis and potentially mild inflammation.[2,3] The current diagnostic criteria for NAFLD require the confirmation of hepatic steatosis through imaging or histological examinations. Importantly, the diagnosis should consider the patient’s daily alcohol consumption, which should not exceed 20 g for women and 30 g for men.[4] Furthermore, it is crucial to effectively rule out all other secondary causes of hepatic steatosis to establish a diagnosis, thereby making NAFLD a diagnosis of exclusion.[5]
Over the past four decades, NAFLD has served as the conventional term for the disease. However, in recent years, the name has been the subject of growing criticism and calls for change. This stems largely from the fact that the definition of NAFLD is inherently “negative,” that is, a diagnosis made by excluding the presence of other conditions.[6] Moreover, the term NAFLD conceals the metabolic roots of the disease, offering limited understanding of its pathophysiological basis. As a result, various alternative names have been proposed, each providing a unique viewpoint and differing degrees of redefinition.[7] Recently, a significant recommendation has emerged, primarily from the Asian Pacific Association for the Study of the Liver, to replace the term NAFLD with metabolic (dysfunction) associated fatty liver disease (MAFLD).[8] This proposed change aimed to shift away from a diagnosis based on the exclusion of other conditions and better reflect the disease’s pathogenesis. The transition from NAFLD to MAFLD represents a more comprehensive approach to provide a “positive” definition of the disease, taking into account the interplay between liver-specific and systemic metabolic factors.[9] The diagnostic criteria for MAFLD are centered around the presence of hepatic steatosis coexisting with any one of these three conditions: (1) Obesity or overweight status, (2) the occurrence of diabetes mellitus, or (3) proven metabolic imbalances as signified by at least two out of seven metabolic risk factors (Table 1) in normal weight individuals (defined as a body mass index of <25 kg/m2 for Caucasians or < 23 kg/m2 for Asians).[8,9] Notably, the introduction of the term MAFLD was anticipated to move away from the dichotomy of NASH and NAFL, focusing instead on evaluating disease severity that includes both inflammation and fibrosis stage.[7–9] Second, it was predicted that MAFLD-related cirrhosis would supersede the outdated term “cryptogenic cirrhosis” for most patients.[7–9] Unfortunately, the suggested renaming of NAFLD to MAFLD sparked a heated debate. The primary critique was that this change could potentially hinder awareness, the establishment of acceptable drug development endpoints, the application of non-invasive scores, and biomarker discovery.[10,11] Under the auspices of the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), Rinella et al.[12] have recently made strides in resolving the ongoing confusion and controversy surrounding the redefinition of NAFLD. Through a meticulous methodology incorporating four Delphi surveys and two face-to-face meetings, they have achieved a consensus on the demanding task of renaming the disease.[12] The term steatotic liver disease (SLD) has been selected as a universal term to cover the diverse causes of hepatic steatosis. SLD can be diagnosed either histologically or through imaging and can stem from various potential causes. Metabolic dysfunction-associated SLD (MASLD) is the term used to describe patients displaying hepatic steatosis who also present at least one of five specific cardiometabolic risk factors (Table 1). Significantly, these risk factors are referred to as cardiometabolic within the MASLD framework, distinguishing them from the metabolic factors identified in the MAFLD framework (Table 1). Furthermore, a new category, MetALD, has been introduced to designate those with MASLD who consume alcohol in higher quantities per week, with the thresholds being 140 g/week for women and 210 g/week for men. In instances where additional factors contributing to steatosis are discovered, it implies a mixed etiology. Where there is a strong suspicion of metabolic dysfunction despite the absence of cardiometabolic risk factors, the term “possible MASLD” may be employed provisionally until more in-depth testing (e.g., oral glucose tolerance test, homeostasis model assessment of insulin resistance) is conducted. Similar cases, along with cryptogenic cases that later exhibit cardiometabolic risk factors, can be reclassified as MASLD. Significantly, the SLD/MASLD framework retains the term steatohepatitis, but replaces NASH with metabolic dysfunction-associated steatohepatitis (MASH).[12] This change ensures that patients previously categorized as NAFLD are now fully encompassed within the MASLD and possible MASLD categories. By maintaining the terminology and clinical definition of steatohepatitis, the continued relevance and applicability of previous clinical trial data and biomarker discovery studies related to NASH is ensured. This is true even for individuals who may be reclassified as MASLD or MASH under the newly proposed nomenclature, without hindering the progress of ongoing research efforts.[12] The likely hesitation of Rinella et al.[12] in embracing the MAFLD definition may stem from the lack of NASH/MASH concept in the initial framework proposed by Eslam et al.[9] Such an omission was perceived to potentially jeopardize ongoing clinical phase studies and biomarker investigations.[10] Regrettably, the struggle for a unified terminology and perhaps, a universal agreement in the field, is likely to persist amongst researchers and clinicians. The “supermajority (67%) vote” as defined by Rinella et al.[12–14] falls short of reaching an all-encompassing consensus. Consequently, the enduring focus of research on NAFLD, a term deeply embedded in history and difficult to eradicate, can be anticipated. There will also be an increased number of studies concentrating on MAFLD and MASLD, some of which may explore the complex distinctions among the two novel terminologies and NAFLD (Fig. 1). Importantly, raising awareness about the complexities of the new classification and its subcategories remains a significant challenge.
Table 1.
Definitions of metabolic (MAFLD framework) and cardiometabolic (MASLD framework) risk factors
| *Definitions of metabolic risk factors within the MAFLD framework |
**Definitions of cardiometabolic risk factors within the MASLD framework |
|---|---|
| Waist circumference ≥102/88 cm or ≥94/80 cm (AHA/NHLBI) in Caucasian men and women or ≥90/80 cm in Asian men and women) | Body mass index ≥25 kg/m2 (23 kg/m2 in Asian individuals) OR waist circumference >94 cm (men) >80 cm (women) OR ethnicity-adjusted values (≥95 cm [men] ≥91 cm [women] in Türkiye) |
| Blood pressure ≥130/85 mm Hg or specific drug treatment | Blood pressure ≥130/85 mm Hg OR specific antihypertensive drug treatment |
| Plasma triglycerides ≥150 mg/dL (≥1.70 mmol/L) or specific drug treatment | Plasma triglycerides ≥150 mg/dL (≥1.70 mmol/L) OR lipid lowering treatment |
| Plasma HDL-cholesterol <40 mg/dL (<1.0 mmol/L) for men and <50 mg/dL (<1.3 mmol/L) for women or specific drug treatment | Plasma HDL-cholesterol ≤40 mg/dL (<1.0 mmol/L) (men) and ≤50 mg/dL (<1.3 mmol/L) (women) OR lipid-lowering treatment |
| Pre-diabetes (i.e., fasting glucose levels 100 to 125 mg/dL [5.6–6.9 mmol/L], or 2-h post-load glucose levels 140–199 mg/dL [7.8–11.0 mmol/L] or HbA1c 5.7–6.4% [39–47 mmol/L]) | Fasting serum glucose ≥ 100 mg/dL [5.6 mmol/L) OR 2-h post-load glucose levels ≥ 140 mg/dL [7.8 mmol/L] OR HbA1c ≥ 5.7% [39 mmol/L]) OR Type 2 diabetes OR treatment for Type 2 diabetes |
| Homeostasis model assessment of insulin resistance score ≥2.5 | |
| Plasma high-sensitivity C-reactive protein level >2 mg/L |
MAFLD: Metabolic (dysfunction) associated fatty liver disease, MASLD: Metabolic dysfunction-associated steatotic liver disease, AHA: American heart association, NHLBI: National heart, Lung, and Blood Institute, HDL: High-density lipoprotein.
Figure 1.
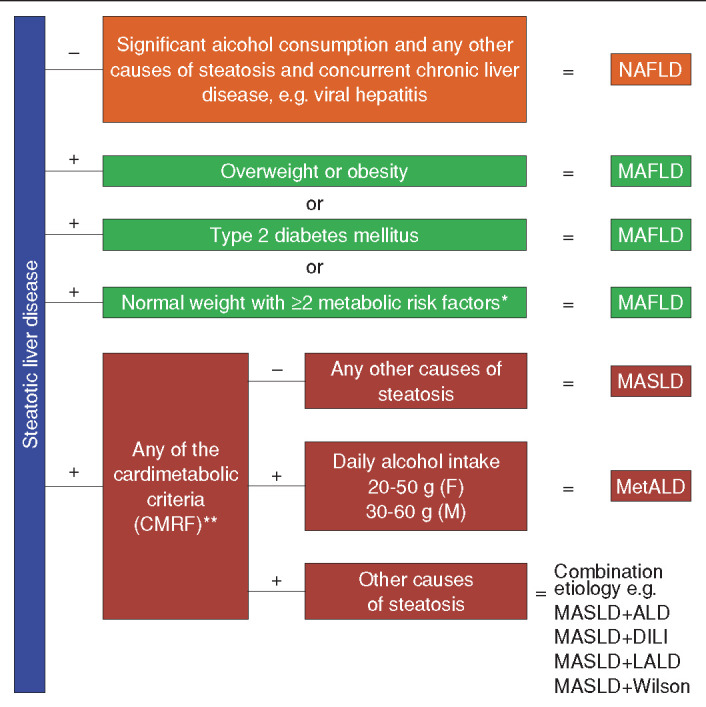
Schematic representation of the diagnostic criteria for NAFLD, MAFLD, and MASLD.
*: Please see the table, **: Please see the table. NAFLD: Non-alcoholic fatty liver disease, MAFLD: Metabolic (dysfunction) associated fatty liver disease, MASLD: Metabolic dysfunction-associated SLD, MetALD: MASLD and increased alcohol intake, ALD: Alcohol-associated (alcohol-related) liver disease, DILI: Drug-induced liver injury, LALD: Lysosomal acid lipase deficiency, CMRF: Cardiometabolik risk factors.
The endeavor to revise a widely recognized acronym like NAFLD is an extensive, complex task, the outcomes of which are currently unpredictable. The ongoing debate between MAFLD/MASLD as potential replacements highlights the inherent challenge in reaching a consensus among different scientific societies, given the divergent viewpoints and stakes involved.[15] The introduction of unfamiliar acronyms only adds to this complexity, potentially leading to confusion or misinterpretation due to their initial lack of recognizability.[16] However, another critical aspect requires attention. While the current discourse primarily engages medical professionals, the inclusion of patients and their families in the renaming process is equally important.[17] They, being the most affected by the disease, can offer invaluable insights into the emotional and personal repercussions of such a name change. The outcomes of this renaming venture are still uncertain, necessitating a cautious approach. To gain a comprehensive understanding of the potential consequences of rebranding NAFLD, it is important to embrace a holistic perspective. This entails conducting thorough research that involves all relevant stakeholders. Such investigations can yield significant insights into the potential impact, both beneficial and detrimental, that such a nomenclature alteration could bring about.
In summary, the ongoing efforts to rename NAFLD are intricate and subject to intense debate, necessitating judicious thought, comprehensive investigations, and the proactive engagement of all relevant parties. It is a venture that demands further scholarly probing to assess implications and ascertain if the advantages of the suggested nomenclature indeed supersede any possible detriments.
Acknowledgement
We would like to express our appreciation to Fatih Kutlu for his valuable contribution in the figure illustration of this article.
Conflict of Interest
Yusuf Yilmaz has served as consultant to Cymabay, Zydus, Novo Nordisk, and Echosens.
Footnotes
How to cite this article: Yilmaz Y. The heated debate over NAFLD renaming: An ongoing saga. Hepatology Forum 2023; 4(3):89–91.
Financial Disclosure
The authors declared that this study has received no financial support.
References
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 2.Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36(9):815–823. doi: 10.1111/apt.12046. [DOI] [PubMed] [Google Scholar]
- 3.Boutari C, Lefkos P, Athyros VG, Karagiannis A, Tziomalos K. Nonalcoholic fatty liver disease vs. nonalcoholic steatohepatitis: pathological and clinical implications. Curr Vasc Pharmacol. 2018;16(3):214–218. doi: 10.2174/1570161115666170621075157. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz Y, Zeybel M, Adali G, Cosar AM, Sertesen E, Gokcan H, et al. TASL practice guidance on the clinical assessment and management of patients with nonalcoholic fatty liver disease. Hepatology Forum. 2023;4(Suppl 1):1–32. doi: 10.14744/hf.2023.2023.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abd El-Kader SM, El-Den Ashmawy EM. Non-alcoholic fatty liver disease: The diagnosis and management. World J Hepatol. 2015;7(6):846–858. doi: 10.4254/wjh.v7.i6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loria P, Lonardo A, Carulli N. Should nonalcoholic fatty liver disease be renamed? Dig Dis. 2005;23(1):72–82. doi: 10.1159/000084728. [DOI] [PubMed] [Google Scholar]
- 7.Yilmaz Y, Byrne CD, Musso G. A single-letter change in an acronym: signals, reasons, promises, challenges, and steps ahead for moving from NAFLD to MAFLD. Expert Rev Gastroenterol Hepatol. 2021;15(4):345–352. doi: 10.1080/17474124.2021.1860019. [DOI] [PubMed] [Google Scholar]
- 8.Eslam M, Sanyal AJ, George J, International Consensus Panel MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 9.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Younossi ZM, Rinella ME, Sanyal AJ, Harrison SA, Brunt EM, Goodman Z, et al. From NAFLD to MAFLD: Implications of a premature change in terminology. Hepatology. 2021;73(3):1194–1198. doi: 10.1002/hep.31420. [DOI] [PubMed] [Google Scholar]
- 11.Méndez-Sánchez N, Zheng MH, Kawaguchi T, Sarin SK. Editorial: The metabolic (dysfunction) associated fatty liver disease (MAFLD)-non-alcoholic fatty liver disease (nafld) debate: a forced consensus and the risk of a world divide. Med Sci Monit. 2022;28:e938080. doi: 10.12659/MSM.938080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023 doi: 10.1097/HEP.0000000000000520. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023:S0168-8278(23)00418-X. doi: 10.1016/j.jhep.2023.06.003. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol. 2023:101133. doi: 10.1016/j.aohep.2023.101133. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Zeng M, Chen L, Li Y, Mi Y, Xu L. Problems and challenges associated with renaming non-alcoholic fatty liver disease to metabolic associated fatty liver disease. Medicine (Baltimore) 2023;3(3):105–113. [Google Scholar]
- 16.Barnett A, Doubleday Z. The growth of acronyms in the scientific literature. Elife. 2020;9:e60080. doi: 10.7554/eLife.60080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon EL, Jun DW. Changing the nomenclature from nonalcoholic fatty liver disease to metabolic dysfunction-associated fatty liver disease is more than a change in terminology. Clin Mol Hepatol. 2023;29(2):371–373. doi: 10.3350/cmh.2023.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]


