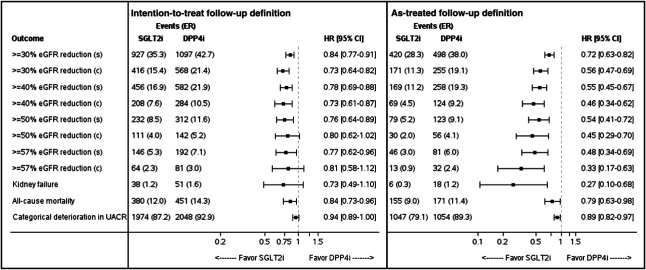
Figure 2.
The association between initiation of sodium-glucose cotransporter 2 inhibitors, compared with dipeptidyl peptidase 4 inhibitors, and other kidney outcomes in the intention-to-treat and as-treated follow-up analyses. In the intention-to-treat analysis, follow-up continued until the occurrence of an effectiveness outcome (for the specific end point), end of data availability, death, or September 2021. The as-treated analysis's follow-up criteria were those used by the intention-to-treat analysis, added by censoring at study drug discontinuation or the initiation of the comparator drug. In the as-treated analysis, follow-up was extended by a grace period of 90 days after the last drug dispensation. Outcomes are assessed either as single (s) or confirmed (c) measurements. Categorical increase in UACR was defined using the following categories: <30, 30–<300, and ≥300 mg/g. Event rates are presented per 1000 patient-year. HR, 95% CIs, and P values were assessed using Cox proportional hazard regression models. ER, event rate; UACR, urine albumin-to-creatinine ratio.