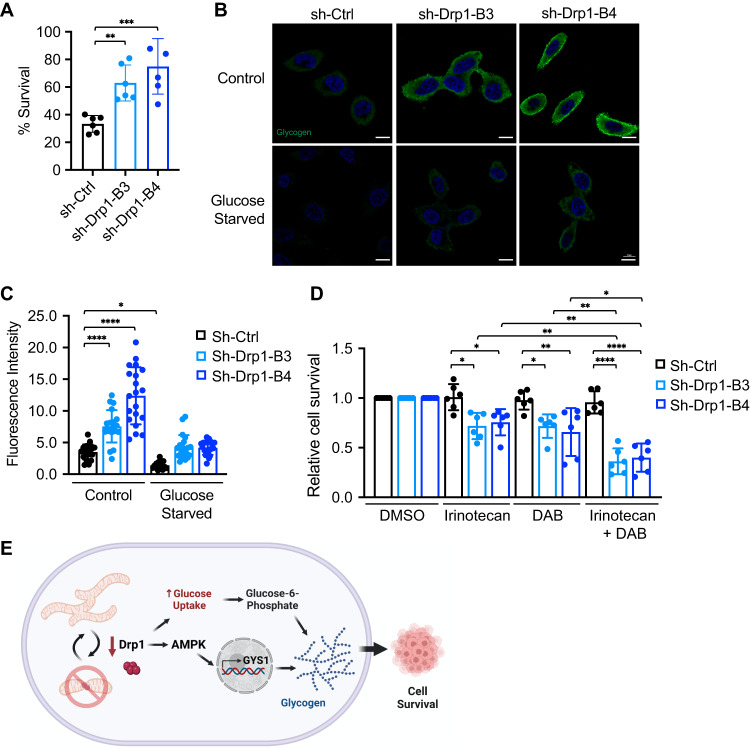
Fig. 6. Increased glycogen storage functions as a survival mechanism in Drp1 knockdown cells.
A Sh-Ctrl and sh-Drp1 PT130 cells were cultured in regular growth media or glucose-free medium for 48 h. The percentage of cell survival were obtained by normalizing the number of cells survived in glucose-free media to that of regular growth media. Data were presented as mean ± SD (n = 6, **p < 0.01 and ***p < 0.001). B Sh-Ctrl and sh-Drp1 PT130 cells were cultured in low glucose or glucose-free media for 24 h. Representative confocal images were obtained from cells stained with the anti-glycogen antibody. Scale Bar, 10 μm. C The relative fluorescence intensity of glycogen staining was quantified using ImageJ fluorescence analyzer. Data were presented as mean ± SD (n = 20, *p < 0.05, **p < 0.01 and ****p < 0.0001). D Sh-Ctrl and sh-Drp1 PT130 cells were treated with irinotecan, DAB or combinations of both agents for 48 h. Cells treated with DMSO were used as control. The relative of cell survival were obtained by normalizing to cells treated with DMSO. Data were presented as mean ± SD (n = 6, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001). E Results from our study demonstrate that disruption of mitochondrial dynamics as a consequence of silencing Drp1 increases glucose uptake and AMPK-dependent transcriptional activation of GYS1. Subsequently, GYS1-mediated glycogen accumulation serves as a compensatory survival mechanism to protect colon cancer cells from glucose starvation and chemotherapy treatment. Thus, co-targeting glycogenolysis may provide a novel strategy to sensitize Drp1 knockdown cells to chemotherapy.