Abstract
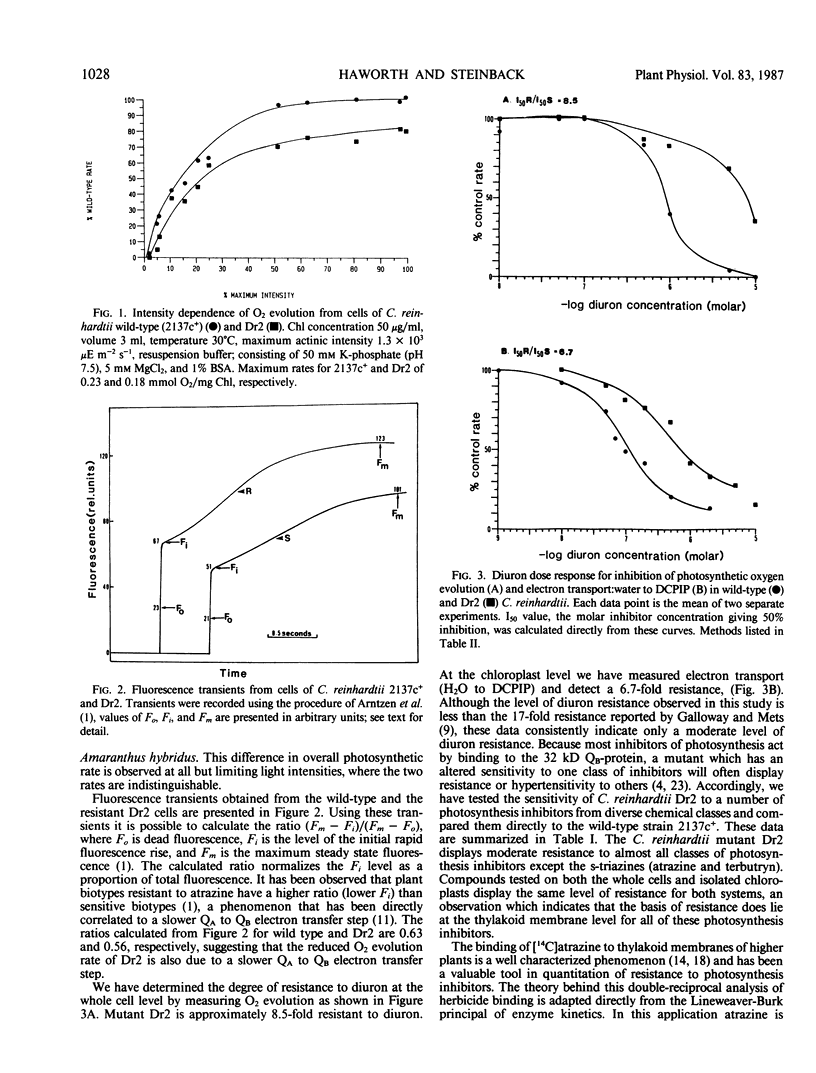
We have used the diuron-resistant Dr2 mutant of Chlamydomonas reinhardtii which is altered in the 32 kilodalton QB-protein at amino acid 219 (valine to isoleucine), to investigate the interactions of herbicides and plastoquinone with the 32 kilodalton QB-protein. The data contained in this report demonstrate that the effects of this mutation are different from those of the more completely characterized mutant which confers extreme resistance to triazines in higher plants. The mutation in C. reinhardtii Dr2 confers only slight resistance to a number of inhibitors of photosynthetic electron transport. Extreme triazine resistance results from an increase in the binding constant of the herbicide with the 32 kilodalton QB-protein, in contrast the diuron binding constant for chloroplasts isolated from wild-type (sensitive) Chlamydomonas and the resistant Dr2 are indistinguishable. We conclude that the altered structure in the 32 kilodalton QB-protein of Dr2 does not directly affect the diuron binding site. This mutation appears to alter the steric properties of the binding protein in such a way that diuron and plastoquinone do not directly compete for binding. This steric perturbation confers mild resistance to other herbicidal inhibitors of photosynthesis and alters the kinetics of QA to QB electron transfer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erickson J. M., Rahire M., Bennoun P., Delepelaire P., Diner B., Rochaix J. D. Herbicide resistance in Chlamydomonas reinhardtii results from a mutation in the chloroplast gene for the 32-kilodalton protein of photosystem II. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3617–3621. doi: 10.1073/pnas.81.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway R. E., Mets L. J. Atrazine, bromacil, and diuron resistance in chlamydomonas: a single non-mendelian genetic locus controls the structure of the thylakoid binding site. Plant Physiol. 1984 Mar;74(3):469–474. doi: 10.1104/pp.74.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway R. E., Mets L. Non-Mendelian Inheritance of 3-(3,4-Dichlorophenyl)-1,1-dimethylurea-Resistant Thylakoid Membrane Properties in Chlamydomonas. Plant Physiol. 1982 Dec;70(6):1673–1677. doi: 10.1104/pp.70.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Ahrens W. H., Martin B., Stoller E. W. Comparison of Photosynthetic Performance in Triazine-Resistant and Susceptible Biotypes of Amaranthus hybridus. Plant Physiol. 1983 Aug;72(4):925–930. doi: 10.1104/pp.72.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Radosevich S. R., Arntzen C. J. Modification of Herbicide Binding to Photosystem II in Two Biotypes of Senecio vulgaris L. Plant Physiol. 1979 Dec;64(6):995–999. doi: 10.1104/pp.64.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional studies with Chlamydomonas reinhardi. Ann N Y Acad Sci. 1953 Oct 14;56(5):831–838. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Stein R. R., Castellvi A. L., Bogacz J. P., Wraight C. A. Herbicide-quinone competition in the acceptor complex of photosynthetic reaction centers from Rhodopseudomonas sphaeroides: a bacterial model for PS-II-herbicide activity in plants. J Cell Biochem. 1984;24(3):243–259. doi: 10.1002/jcb.240240306. [DOI] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]


