Abstract
Post-acute COVID-19 sequelae, commonly known as long COVID, encompasses a range of systemic symptoms experienced by a significant number of COVID-19 survivors. The underlying pathophysiology of long COVID has become a topic of intense research discussion. While chronic inflammation in long COVID has received considerable attention, the role of neutrophils, which are the most abundant of all immune cells and primary responders to inflammation, has been unfortunately overlooked, perhaps due to their short lifespan. In this review, we discuss the emerging role of neutrophil extracellular traps (NETs) in the persistent inflammatory response observed in long COVID patients. We present early evidence linking the persistence of NETs to pulmonary fibrosis, cardiovascular abnormalities, and neurological dysfunction in long COVID. Several uncertainties require investigation in future studies. These include the mechanisms by which SARS-CoV-2 brings about sustained neutrophil activation phenotypes after infection resolution; whether the heterogeneity of neutrophils seen in acute SARS-CoV-2 infection persists into the chronic phase; whether the presence of autoantibodies in long COVID can induce NETs and protect them from degradation; whether NETs exert differential, organ-specific effects; specifically which NET components contribute to organ-specific pathologies, such as pulmonary fibrosis; and whether senescent cells can drive NET formation through their pro-inflammatory secretome in long COVID. Answering these questions may pave the way for the development of clinically applicable strategies targeting NETs, providing relief for this emerging health crisis.
Keywords: neutrophils, neutrophil extracellular traps, COVID-19, long covid, thrombosis, autoimmunity, fibrosis, inflammation
1. Introduction
Long COVID (LC), also called post-acute sequelae of SARS-CoV-2 infection, encompasses the persistent symptoms following COVID-19. The Centre for Disease Control and Prevention (CDC) defines LC as symptoms lasting beyond four weeks post-SARS-CoV-2 infection, while the World Health Organization (WHO) describes it as the continuation or development of new symptoms three months following the initial infection, lasting for at least 2 months without any alternative explanation. LC is a multisystem disorder encompassing a multitude of clinical presentations including respiratory and cardiac dysfunction, neuropsychiatric disturbances, and hypercoagulability (1–3).
The prevalence of LC is rising, with an estimated 65 million people affected. This comprises 10-30% of non-hospitalized cases, 50-70% of hospitalized cases, and 10-12% of vaccinated-infected individuals (1–3). Hence, COVID-19 patients of all severities appear to be affected by LC. However, these figures likely underestimate the true prevalence and incidence of LC due to underreporting and undocumented cases. A substantial proportion of LC patients report significant impairments in their quality of life with an inability to perform tasks of daily living (4). Unfortunately, no effective treatment exists for LC, with a need for further high-quality and methodologically robust clinical trials (5). Viable therapeutic targets for long COVID are also lacking, partly due to the multitude of pathophysiological mechanisms that have been identified and a poor understanding of which of these mechanisms is causally linked to LC versus those that simply modulate its biology. Nevertheless, theories of LC pathophysiology converge on a chronic inflammatory response involving both the innate and adaptive arms of host defense (6, 7).
Neutrophils, the most abundant circulating leukocytes, constitute an essential component of innate immunity and are the first responders to sites of acute inflammation. They contribute to host defense by phagocytosis, pro-inflammatory cytokine production, degranulation, and the formation of neutrophil extracellular traps (NETs) (8, 9). Exaggerated neutrophil responses, particularly the production of NETs, have been implicated in the pathogenesis of acute COVID-19. NETs are web-like structures composed of cytosolic and granular neutrophil proteins embedded in a meshwork of either nuclear or mitochondrial neutrophil DNA (10–12). Various strategies targeting NETs have emerged as potential approaches to alleviate their pathological effects in COVID-19 and other autoimmune and inflammatory disorders (13–16).
Studies have indicated that the persistent release or impaired clearance of NETs after COVID-19 resolution may be linked to the pathogenesis of LC. This review explores the potentially disease-mediating role of NETs in the multi-faceted pathophysiology of LC.
2. Neutrophil extracellular traps
2.1. Production
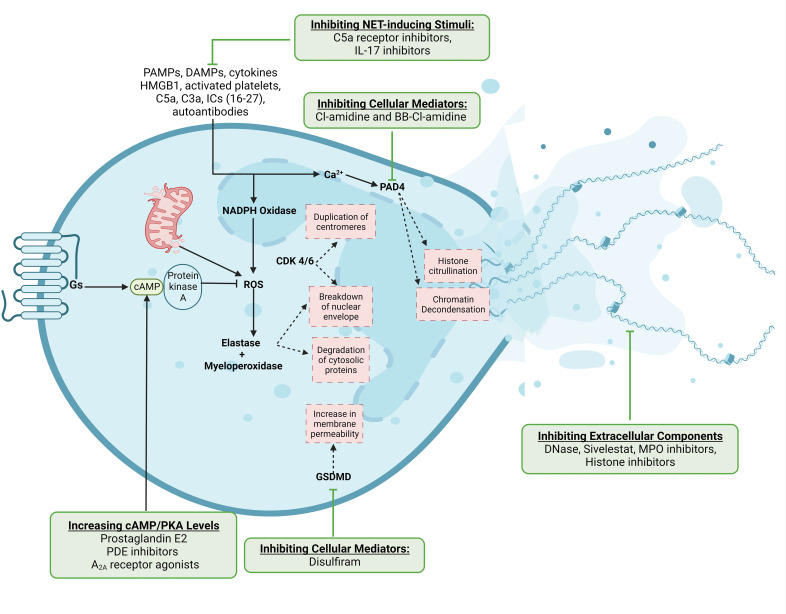
NET production, traditionally referred to as NETosis, was initially believed to occur exclusively through the pyroptotic cell death of neutrophils, resulting in the extrusion of nucleic acids and proteins into the extracellular space ( Figure 1 ). Through a process called vital NET formation, however, neutrophils can release their contents and remain viable thereafter.
Figure 1.
Mechanisms of NET production and potential therapeutic targets. NET-inducing stimuli converge on the production of reactive oxygen species, which activate neutrophil proteases and enzymes to cause downstream nuclear envelope breakdown and chromatin decondensation. The release of NETs either involves cell death with the extrusion of DNA-bound proteins or the exocytosis of NET components through the cell membrane from viable neutrophils. We have also indicated steps in this pathway which have been therapeutically targeted. This Figure was created using BioRender.com.
Various stimuli have been reported to induce NET formation, including microbial pathogen-associated molecular patterns (PAMPs) and their virulence factors, pro-inflammatory cytokines, damage-associated molecular patterns (DAMPs) like high-mobility group box-1 (HMGB1) and ATP, activated platelets, complement proteins C5a and C3a, autoantibodies, and immune complexes (17–28). These stimuli converge on increasing cytosolic calcium concentrations, leading to the activation of NADPH oxidase and the generation of reactive oxygen species (ROS) like superoxide and hydrogen peroxide—a process termed the respiratory burst (29). ROS molecules activate neutrophil proteases such as neutrophil elastase (NE) and myeloperoxidase (MPO) (30). Mitochondrial ROS can trigger similar processes but in a NAPDH oxidase-independent manner (31). NE and MPO then degrade the nuclear envelope, cleave numerous cytosolic proteins, and de-condense nuclear chromatin (30). The cell cycle proteins cyclin-dependent kinases 4 and 6 (CDK4/6) can also be repurposed to aid in nuclear envelope breakdown (32). Proteolytic cleavage of the pore-forming protein gasmerdin D (GSDMD) by caspases, NE, and MPO results in its activation and subsequent translocation to the cell membrane, increasing its permeability and augmenting the intracellular calcium flux (33, 34). Concomitantly, the activation of peptidylarginine deaminase-4 (PAD4), a calcium-dependent enzyme, catalyzes histone citrullination, disrupting the electrostatic attractions between DNA and histones and leading to chromatin decondensation (35). Ultimately, chromatin disassembly and compromised cell surface and nuclear membrane integrity lead to the release of DNA and proteins as NETs.
There is no singular marker for NETs, but extracellular DNA, NE, MPO, and citrullinated histone (cit-H3) are frequently used as surrogate markers. It is worth noting that numerous controversies have arisen regarding the exact relevance of several of the above-mentioned mediators. For instance, NET production in response to inflammasome signaling was previously thought to involve GSDMD-dependent processes (36), but recent GSDMD knockout experiments suggest a dispensable role of GSDMD in NET production (37). Similar results have been observed in NADPH and PAD4 knockout experiments (38, 39). These findings indicate that redundant mechanisms of NET production exist and call into question the exact functional relevance of many of these proteins and, by extension, their therapeutic value. Idiosyncratic, stimulus-dependent mechanisms likely exist, the elucidation of which may enable context-specific therapeutic manipulations. These controversies and others are covered in more detail elsewhere (see (40)).
From a metabolic standpoint, neutrophils have relatively few mitochondria compared to lymphocytes and macrophages and so rely predominantly on ATP acquired through glycolysis (41). The extrusion of NETs appears to also be majorly dependent on glycolysis, such that stimulating NET production in vitro by PMA under low glucose conditions causes neutrophils to lose their characteristic polymorphic nuclear architecture but does not result in the extrusion of NETs (42). Similarly, conditional deletion of the mitochondrial protein optic atrophy-1 (OPA1) in mice inhibits mitochondrial complex I activity, which decreases NAD+ availability for the glycolytic pathways and hence inhibits NETosis, with these models consequently displaying a subdued antibacterial response to Pseudomonas aeruginosa infections (43). It was later described that the activity of lactate dehydrogenase (LDH) and lactate production are essential for NET release, and the mechanism by which PMA induces NETosis involves LDH induction (44). Accordingly, sodium oxamate, an LDH inhibitor, attenuates NET production in mouse models of sepsis (44). Extracellular acidosis was recently shown to inhibit glycolysis and lactate production, thereby attenuating NETosis (45). The metabolic regulation of NET production seems, therefore, to be strictly dependent on glycolysis and lactate production and can be perturbed in systemic conditions where extracellular nutrient and metabolite levels are altered. However, whether therapeutically manipulating neutrophil metabolism can alter NETosis and allow us to modulate the pathogenesis of various disease states is not understood.
2.2. Therapeutic targets
Several mediators involved in NET production constitute therapeutic targets ( Figure 1 ). For simplicity, we can divide NET-targeting strategies into four groups (1): inhibiting NET-inducing stimuli, such as pro-inflammatory cytokines, platelets, DAMPs like HMGB1, and complement proteins (2); inhibiting cellular mediators of NETosis, including ROS, PAD4, and GSDMD (3); the extracellular effectors of NETs, including NE, MPO, and histone inhibitors; and (4) augmenting the degradation of NETs through DNase-1. There are drugs in clinical use that inhibit NET-inducing stimuli, such as C5a receptor antibodies for ANCA-associated vasculitides and IL-17 inhibitors for psoriasis (46, 47). Other than these drugs, however, translational research on many of the other targets is lacking, and the ones that have been tested often lack a substantial clinical benefit. For example, the NE inhibitor sivelstat failed to significantly improve the outcomes of patients in clinical trials (48). The PAD4 inhibitors Cl-amidine and BB-Cl-amidine have shown promise in several animal models but lack human data (49–51). Dnase-1, which is FDA-approved for use as an inhalant in cystic fibrosis (CF) patients (52), is the only drug specifically targeting NETs approved for use. Recent studies have demonstrated the benefit of DNase-1 in improving outcomes of hospitalized patients with COVID-19-related ARDS, although validation in larger randomized studies is awaited (53–55). Disulfiram, commonly used for alcohol use disorder, can inhibit GSDMD pore formation and reduce NET burden and fibrosis in hamsters with COVID-19 (56, 57), but it has not been clinically studied for this indication. The cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling pathway also suppresses NET production by inhibiting the oxidative burst. Therapeutically, this pathway can be leveraged by prostaglandin E2, cAMP analogs, phosphodiesterase inhibitors, and adenosine A2A receptor agonists to increase intracellular cAMP levels and suppress NETosis (58–60). Overall, the COVID-19 pandemic has highlighted the abundance of potential targets for NET inhibition, but a glaring paucity of applicable medications whose clinical benefit has been substantiated.
3. Neutrophils in acute COVID-19
3.1. SARS-CoV-2 infection
SARS-CoV-2 is an enveloped positive-sense single-stranded RNA virus with a helical nucleocapsid belonging to the coronavirus family. Its viral genome encodes four structural proteins, including a surface spike (S) protein, which facilitates viral cell entry and membrane fusion (61). The S protein is composed of two subunits, S1 and S2. The S1 subunit contains the receptor-binding domain (RBD) which binds to angiotensin-converting enzyme 2 (ACE2) on host cell surfaces initially of the nasopharyngeal epithelium (62, 63). This initial interaction is established in the upper respiratory tract and is followed by subsequent seeding to the lower respiratory tract (64).
After binding to ACE2, the S protein undergoes cleavage by the transmembrane protease serine 2 (TMPRSS2) (62). Cleavage exposes the fusion peptide of the S2 subunit, resulting in the fusion of viral and host cell membranes (65). Following successful cellular infection, essential viral proteins are synthesized, and progeny SARS-CoV-2 virions are released via exocytosis to infect other cells. Type II pneumocytes are the primary ACE2-expressing cells in the alveolar epithelium, hence serving as the primary target of SARS-CoV-2 infection in the lower respiratory tract (66, 67).
Tissue-resident alveolar macrophages represent the first line of innate immune defense against SARS-CoV-2, recognizing SARS-CoV-2 PAMPs through various pattern-recognition receptors (PRRs), and triggering the production of pro-inflammatory cytokines and chemokines (68–70). This initial innate response is crucial in determining the severity of COVID-19 (71, 72). The timely production of type I and III interferons and cytotoxic T-cell responses is associated with mild-to-moderate infection (73–75). In contrast, severe disease arises from an impaired interferon and T-cell response coupled with an exaggerated innate neutrophil response (73–75). This dichotomy in the immune response plays a pivotal role in determining the severity of COVID-19 (71, 72). Clinically, the majority (~80%) of individuals infected with COVID-19 experience either asymptomatic or mild disease, with around 15% necessitating oxygen support, while 5% develop septic shock, ARDS, and multi-organ failure requiring aggressive treatments in intensive care units (ICUs) (76).
3.2. Neutrophil responses in COVID-19
Any discussion on the role of NETs in a specific disease is incomplete without describing the contextualized perturbations observed in the cells responsible for their production. This section delves into the current knowledge surrounding numerical and functional changes in neutrophils that ensue after SARS-CoV-2 infection. We focus on three key aspects: neutrophil alterations in the circulation, bronchoalveolar lavage (BAL), and histopathologic examination of lung autopsies from deceased COVID-19 patients.
Neutrophilia with an elevated neutrophil-to-lymphocyte ratio is a salient feature of severe COVID-19 (77). The degree of neutrophilia correlates positively with disease severity, being the highest in severe cases. Nonetheless, neutrophilia in COVID-19 is orders of magnitude less pronounced than that seen in bacterial ARDS (78). Notably, circulating neutrophils isolated from individuals with severe COVID-19 exhibit pronounced activated phenotypes (79), indicating that the disease not only induces numerical alterations but also significantly impacts the phenotype of the neutrophil compartment.
Recent research utilizing single-cell analysis has shed light on the transcriptional diversity of neutrophil populations (80, 81). A subset that has attracted considerable attention is interferon (IFN)active neutrophils, which are thought to be primed for infection control (82). These subsets also appear in COVID-19, but their exact significance is still unclear (discussed below). Another way of classifying neutrophils is based on their density into low-density neutrophils (LDNs) and normal-density neutrophils (NDNs). LDNs are functionally heterogeneous groups of neutrophils but are generally considered more immature cells that produce higher levels of pro-inflammatory cytokines and NETs (83), with their emergence in autoimmune disorders like systemic lupus erythematosus (SLE) being associated with disease pathogenesis and severe clinical phenotypes (84, 85).
In the circulation, analyses of whole blood and PBMCs have demonstrated the emergence of immature neutrophil populations in severe—but not mild—COVID-19 (86, 87). This finding indicates the activation of emergency myelopoiesis, although the presence of immature neutrophils is not as significant as in cases of bacterial ARDS (78). Both immature and mature neutrophils display transcriptional signatures consistent with an activated state, characterized by upregulated calprotectin (i.e., S100A8/A9) expression and enhanced NET production (78, 87–89). Severe COVID-19 is also associated with the expansion of immunosuppressive clusters of neutrophils expressing the immune checkpoint molecule PD-L1 (87, 90–92), which declines in individuals recovering from severe disease. Another immunomodulatory cluster of neutrophils associated with severe disease are IFNactive neutrophils (87, 92), which are depleted by dexamethasone treatment, suggesting their involvement in the immunopathogenesis of severe COVID-19 (92). However, IFNactive neutrophils also occur in mild COVID-19, which is part of a coordinated interferon-stimulated gene (ISG) response across all major immune cell populations in the blood, whereas these ISG-expressing cells are systematically absent in patients with severe disease (93), consistent with the globally blunted type I and III IFN response characteristic of severe COVID-19 (73–75). These findings indicate that the presence of IFNactive neutrophils is not a necessary component of severe disease. The topic of neutrophil heterogeneity in COVID-19 has been detailed by other reviews (94, 95). Nonetheless, many of the current studies on this topic infer distinct functionality from the transcriptomic data rather than directly observing whether distinct subsets differentially impact COVID-19 pathogenesis. Subset-specific manipulations are required to further current findings.
Findings regarding neutrophil numbers and phenotypes in bronchoalveolar lavage (BAL) are largely concordant with those observed in the circulation. Numerically, mild COVID-19 does not exhibit a significant neutrophilic infiltrate, which progressively increases with worsening disease severity (96–98), surpassing even the levels seen in bacterial ARDS (86, 99). Phenotypically, BAL neutrophils exhibit similar activation signatures-such as calprotectin expression and NET production-as their circulating counterparts (100). However, it remains unclear whether specific transcriptional clusters of circulating neutrophils are selectively recruited to the lungs or whether they infiltrate the infection site indiscriminately. ScRNA-seq of BAL samples identified 5 transcriptionally distinct neutrophil clusters in severe COVID-19 patients, particularly those expressing VEGFA, chemokine receptors, S100 proteins, and IFNs, which differentially impact COVID-19 severity depending on interactions with other immune cells (101). Mild COVID-19 is characterized by a robust response of debris-clearing monocytes and anti-viral T-cells, with IFNactive neutrophils being involved in viral clearance. Conversely, severe infection is characterized by a depletion of monocytes and T-cells that keep neutrophils in check, resulting in uncontrolled neutrophilic responses characterized by the overwhelming production of pro-inflammatory alarmins (e.g., S100A8/A9) and NETosis (101).
Histological examinations of lung autopsies from patients who succumbed to SARS-CoV-2 have corroborated these findings, revealing the dense presence of alarmin- and NET-producing neutrophils (102, 103). Notably, S100A8/A9 is key to aberrant neutrophil responses in COVID-19, driving exaggerated innate immune inflammation and uncontrolled pathological damage (104). Inhibition of S100A8/A9 has shown promise in attenuating COVID-19-related pneumonia in experimental models (104).
These findings provide valuable insights into the complexities of neutrophil responses in the pathogenesis of COVID-19. Further investigations are warranted to understand the functional impact of distinct neutrophil subsets and specific properties that have potential as therapeutic targets.
3.3. Neutrophil extracellular traps in acute COVID-19
The involvement of NETs in acute COVID-19 was hypothesized early in the pandemic (105–107) and subsequently confirmed by seminal studies conducted by Zuo et al. and Middleton et al. (79, 108). These investigations documented that plasma MPO-DNA complexes were found to be elevated in COVID-19 patients and correlated with disease severity, as evidenced by a higher sequential organ failure assessment (SOFA) score, incidence of intubation and death, and lower PaO2/FiO2 ratios (79, 108). Studies that followed expanded on the prognostic role of NETs, with extracellular DNA, cit-H3, and NE also being associated with clinical events and ICU admission (109, 110). Neutrophils isolated from the plasma of COVID-19 patients exhibit heightened basal NETosis, suggesting a predisposition towards NET formation (79). Furthermore, the serum of COVID-19 patients induced NETosis in control neutrophils (79), highlighting the systemic activation state induced by the virus.
A striking feature of severe COVID-19 lung disease that sets it apart from other viral pneumonia is the presence of distinctive vascular features, including widespread thrombosis and microvascular occlusion with secondary lobular ischemia (111). These vascular abnormalities are associated with endothelial activation and damage, which, in turn, trigger inflammation and thrombosis. Histopathological examination of post-mortem lung specimens from COVID-19 patients has revealed a dense neutrophilic infiltrate, neutrophil-platelet interactions, and the presence of NETs in pulmonary microthrombi (79, 102, 112, 113). These observations suggest that NETs contribute to the coagulopathy observed in severe COVID-19. Mechanistically, the web-like structure of NETs acts as a scaffold for the deposition of various clotting factors, promoting coagulation (114). Additionally, extracellular histones in NETs activate endothelial cells and platelets via TLR2 and TLR4 (115–119), and NET-associated proteases cleave and inactivate tissue factor pathway inhibitor, augmenting tissue factor activity (120). Skendros et al. demonstrated that neutrophils release NETs enriched in tissue factor, contributing to thrombosis in COVID-19 ARDS (121). Other than this study, the mechanisms by which certain NET components contribute to COVID-19 pathogenesis have been extrapolated from non-COVID studies; direct evidence elucidating the specific pathogenic roles of these components in COVID-19 is scare in contemporary literature.
Multiple stimuli of NETosis in COVID-19 have been identified. There is evidence that SARS-CoV-2 can directly infect neutrophils through ACE2 and TMPRSS2, triggering NET formation (121). Platelets are a major inducer of NETs, as exposing control neutrophils to platelet-rich plasma from COVID-19 patients is sufficient to stimulate NETosis (121). Platelets, which do not typically interact with neutrophils, become important inducers of NETs in the context of bacterial and viral infections (22, 122, 123), perhaps mediated by platelet factor-4 (PF4) (103), p-selectin or high-mobility group box 1 (HMBG1) (119). Autoantibodies against PF4, observed in severe COVID-19 and vaccine-induced thrombotic thrombocytopenia (VITT), are also capable of stimulating NET formation (124–126). Lastly, elevated levels of complement proteins, particularly C3 and C5, in COVID-19 provide another potential pathway for NETosis (121, 123, 127).
To gain a deeper understanding of NETs in COVID-19, future studies employing higher-resolution approaches are needed. Genetic or pharmacologic loss-of-function experiments targeting specific NET components could provide valuable insights into their precise pathogenic effects. It is important to consider the dichotomous nature of NETs, as they have been shown to play both beneficial and pathologic roles in various contexts. For instance, NETs mitigate the systemic spread of HIV-1 and Chikungunya virus infection (128, 129), with DNase-1 administration leading to disseminated infection (129). NETs can also promote healing through the degradation of pro-inflammatory molecules by their proteases (130). Importantly, degrading NETs as a therapeutic strategy can inadvertently worsen the cytotoxic/pro-inflammatory/pro-thrombotic effects of NET-bound DNA and histones when they are liberated from NETs as NET-degradation products (131, 132). NETs can suppress pro-inflammatory macrophage phenotypes in autoimmune disorders like rheumatoid arthritis (133), and induce anti-inflammatory M2 polarization in Leishmania parasite infection (134) and myocardial infarction (135), associated with decreased tissue damage in the latter. The balance between these dual roles may reflect factors such as the origin of neutrophil subsets, the stimuli triggering NET formation, the degree of neutrophil activation, and the composition of NETs themselves. Therefore, comprehensive investigations into NETs in COVID-19 are crucial for discerning their precise contributions and facilitating more precise therapeutic manipulations.
4. Neutrophils and NETs in chronic lung disease
Investigations into the association between neutrophil responses, NETs, and LC have predominantly focused on chronic lung disease and pulmonary fibrosis. The discussion herein discusses the role of neutrophils and NETs in chronic lung inflammation and pulmonary fibrosis in a myriad of non-COVID diseases.
4.1. NETs in chronic inflammation
NETs have emerged as pivotal contributes towards several chronic lung diseases, including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, and bronchiectasis, where they damage host tissue, impair mucociliary clearance, hinder bacterial cell killing, and exacerbate inflammation (136). Elevated neutrophil counts and NET markers, such as cell-free DNA, have been observed in the lungs and sputum of COPD and asthma patients, demonstrating a positive correlation with disease severity and the frequency of exacerbations (137–140). The release of the inflammasome-related cytokine IL-1β by macrophages can induce NETosis in asthma (141). NET contents components such as DNA, LL-37, defensins, and NE are pro-inflammatory and induce the release of histamine and leukotrienes, leading to worsening disease severity (136, 142).
Cystic fibrosis patients experience recurrent infections and have a lung microenvironment rich in pro-inflammatory cytokines and chemokines that promote neutrophil infiltration and NET production (143). The sputum of cystic fibrosis patients exhibits higher levels of extracellular DNA, NE, MPO, and calprotectin (144–146). Among these, DNA and NE are particularly associated with declining lung function and increased disease severity (147–149). Additionally, the virulence factor pyocyanin produced by Pseudomonas aeruginosa—the primary cause of pneumonia in cystic fibrosis patients—can enhance NET formation (150, 151). DNase is an effective therapy in reducing mucus viscosity in CF patients by degrading NET-derived DNA (52). However, the double-edged role of NETs is evident here, with NET degradation recently shown to liberate NET-bound NE, enhancing its activity and facilitating tissue damage (152).
Bronchiectasis is characterized by non-resolving neutrophilic inflammation and is marked by elevated levels of NETs in sputum, with their levels positively correlated with exacerbation incidence, worsening lung function, and mortality (153). Treating active infections or reducing inflammation in bronchiectasis patients reduces NET levels, leading to clinical improvement (154). Moreover, emerging evidence suggests that antibiotics such as macrolides can directly attenuate NETs independent of their antimicrobial effects (154).
Together, these findings underscore the role NETs play in lung diseases featuring chronic inflammation. Mechanistically, NET components contribute to the amplification of pro-inflammatory cytokines and chemokines, including IL-1, IL-6, and IL-8, by alveolar epithelial cells and alveolar macrophages, thereby perpetuating repetitive cycles of inflammation and alveolar epithelial damage (155, 156). Smoking-related airway disease exemplifies these features, as smoking induces the release of NETs, which, in turn, promote T-cell differentiation and pro-inflammatory cytokine secretion by airway epithelial cells and macrophages, ultimately fostering chronic airway disease (157, 158).
NETs also damage the alveolar epithelium in various models of acute lung injury, including transfusion-associated lung injury (159), trauma (160), ventilator-associated lung injury (161), primary graft dysfunction after lung transplantation (162), lipopolysaccharide-related sepsis (163), influenza pneumonitis (164), and COVID-19 (79, 108, 165, 166), all of which are known to feature fibrosis as a sequela. A recent review by Scozzi and colleagues details the role of NETs in these diseases (167). The induction of NETs leads to cytotoxic effects on the alveolar epithelium and vascular endothelium (146, 160, 168). Additionally, NETs contain tissue factor, which contributes to thrombosis in ARDS (169).
4.2. NETs in fibrosis
Interstitial lung disease (ILD) is an umbrella term that encompasses a range of conditions characterized by diffuse lung parenchymal changes, alveolar inflammation, and interstitial fibrosis. ILD can result from various causes, including environmental (pneumoconiosis, hypersensitivity pneumonitis), smoking, drugs (bleomycin, chemotherapy, nitrofurantoin), connective tissue disease (SLE, scleroderma), or idiopathic origins (IPF vs non-IPF). This discussion focuses on specific pathways through which NETs contribute to fibrosis, shedding light on potential mechanisms that may also translate in the context of LC.
Lung fibroblasts, which constitute the majority of the cellular constituents of the lung parenchyma, are the major effectors of pulmonary fibrosis (170). Several NET components have been associated with fibroblast activation (170). Myofibroblasts exposed to NETs display increased expression of connective tissue growth factors, collagen production, and proliferation/migration, with these effects being mitigated by DNase-1 treatment (171). Bleomycin, a drug known to induce pulmonary fibrosis, triggers PAD4-dependent NET formation in neutrophils, contributing to the development of ILD. Accordingly, PAD4-KO mice display attenuated bleomycin-induced NETosis with decreased pulmonary fibrosis (172).
NET components such as histone H3 and MPO promote the differentiation of lung fibroblasts into myofibroblasts, an effect that is attenuated by exogenous DNase-1 administration (173). NE within NETs has recently been shown to induce fibroblast proliferation and myofibroblast differentiation, thereby facilitating the progression of lung fibrosis (174). Notably, NE-KO mice were protected from asbestos-induced lung fibrosis (174). NE has also been implicated in promoting α-SMA and fibronectin expression in macrophages, resulting in a fibrotic phenotype termed the macrophage-to-myofibroblast transition (175, 176). Additionally, IL-17 contained within NETs enhances the fibrotic activity, but not the differentiation, of myofibroblasts, suggesting that the NET components mentioned earlier may prime the process of IL-17-driven fibrosis (171).
Epithelial-to-mesenchymal transition (EMT), induced by NETs, has been observed in LC and is a widely recognized phenomenon in various disease contexts. Chronic lung allograft dysfunction (CLAD), characterized by marked lung fibrosis and subsequent dysfunction, is associated with NET production, which triggers EMT in alveolar epithelial cells, as evidenced by increased α-SMA and decreased E-cadherin expression, closely resembling the effects of the well-known EMT inducer TGF-β (177). Endothelial cells can internalize NETs through their surface RAGE receptors (178), but the persistent NET release overwhelms their uptake capacity, leading to the accumulation of NETs in the extracellular space. Subsequently, NE disrupts intercellular tight junctions to increase vascular permeability and promotes endothelial-to-mesenchymal transition by promoting β-catenin signaling (178). Macrophages, also through RAGE receptors, can internalize NETs (176). The phagocytosis of NE into macrophages has been shown to induce the macrophage-to-mesenchymal transition in murine models of post-spinal surgery fibrosis (176). Similarly, NETs facilitate the macrophage-to-myofibroblast transition and attenuate TGF-β1 secretion from macrophages, promoting renal fibrosis after unilateral renal obstruction and the fibrotic remodeling of chronic venous thrombi (179, 180).
5. Neutrophil extracellular traps in long covid
5.1. The theorized role of NETs in long COVID
Sawadogo et al., drawing upon knowledge of NETs and their interaction with the adaptive immune system, hypothesized a link between prolonged neutrophil activation, NET release, and the development of LC (181). They highlighted the fact that NET components—including double-stranded DNA, histones, citrullinated peptides, MPO, and proteinase-3—are unbeknownst to the adaptive immune system and, hence, constitute neoantigens. These neoantigens have the potential to initiate and sustain autoimmune processes by triggering the production of autoantibodies (14, 182). Compelling evidence from conditions like systemic lupus erythematosus and rheumatoid arthritis shows that the presence of autoantibodies can induce NETosis and subsequently protect them from degradation, supporting the notion that NETs can foster autoimmunity by harboring neoantigens (182). This phenomenon would fuel a chronic pro-inflammatory response, activation of the coagulation cascade, and fibrosis (40, 82). Similar to SLE, COVID-19 exhibits the presence of LDNs in circulation, which release NETs enriched with oxidized nucleic acids that possess heightened immunostimulatory capabilities, further enhancing autoimmunity and IFN responses (27). Our group similarly postulated a role for NETs-related autoimmune vasculitides as a mechanism of LC vascular disease (183).
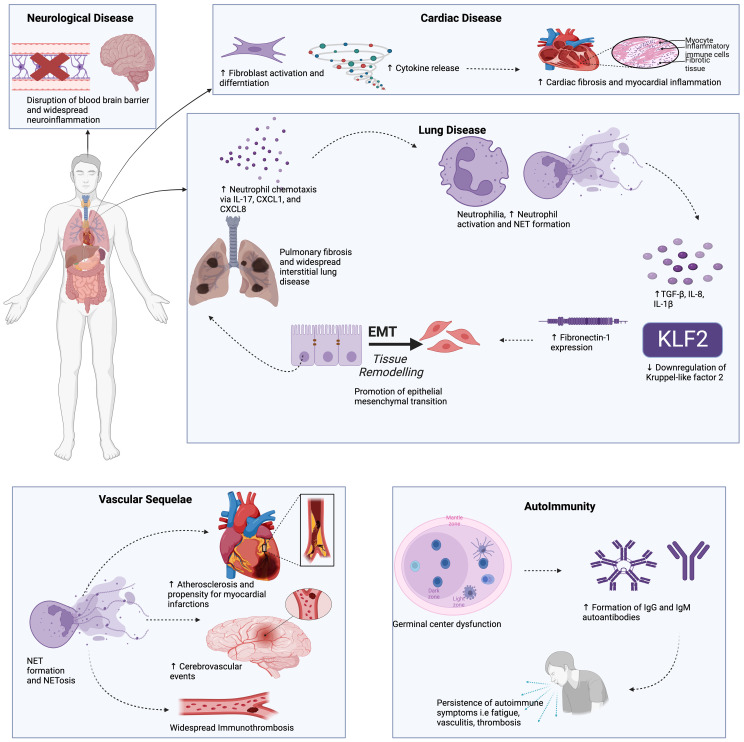
The discussion below focuses on the role of NETs in autoimmunity, lung disease, cardiovascular disease, and neurologic/neuropsychiatric complications seen in LC ( Figure 2 ). We also present emerging data showing cellular senescence-associated inflammation to play a role in LC pathogenesis.
Figure 2.
The potential role of NETs in long COVID may involves multiple pathways. NETs have been shown to drive pro-fibrotic responses in the alveolar epithelium and lung fibroblasts, leading to lung fibrosis. An array of autoantibodies detected in long COVID, including anti-NET IgM and IgG, which have been shown to protect NETs against degradation, contributing to downstream inflammation, vascular damage, and thrombosis. NETs are known to contribute to atherosclerosis and myocardial inflammation, perhaps explaining the cardiovascular sequelae in long COVID patients. Neuropsychiatric abnormalities in long COVID are associated with neuroinflammation and blood-brain barrier disruption, which can be induced by neutrophil-endothelial cell interactions and NET production. This Figure was created using BioRender.com.
5.2. NETs and autoantibodies in long COVID
Studies have demonstrated that circulating neutrophils isolated from individuals with LC display higher levels of NET induction compared to those of healthy controls (184, 185). Longitudinally following surrogate NET markers for at least 6 months in patients previously hospitalized for COVID-19 demonstrates sustained elevations in serum concentrations of NE, MPO, and cell-free DNA, although they were lower compared to acute COVID-19 patients but still higher than non-COVID controls (186). These findings indicate an incomplete resolution of NETs after acute COVID-19.
Severe COVID-19 features the emergence of autoantibodies against a wide array of proteins including type I IFNs, numerous interleukins, and self-antigens associated with autoimmune diseases, such as anti-nuclear antibodies (ANAs), anti-histone antibodies, and anti-neutrophil cytoplasmic antibody (ANCA), which are components of NETs (187–190). Most of the autoantigens identified exist complexed to extracellular nucleic acids—and such would be the case within NETs—which are then recognized by nucleic acid PRRs such as Toll-like receptors (e.g., TLR7) (189). Although the exact mechanism of their pathogenicity is unclear, their presence is positively correlated with disease severity. However, a large cohort study comparing non-ICU and ICU-admitted COVID-19 patients demonstrated that ANAs are not associated with disease severity but rather reflect a dysregulated immune response due to extensive cell death (191).
Importantly, the detection of IgG and IgM autoantibodies against NETs is frequent in hospitalized COVID-19 patients, their levels tracking with increased circulating NET markers and worse disease outcomes (192). The levels of anti-NET IgG inversely correlate with the ability to clear NETs, suggesting that autoantibodies protect NETs from degradation by DNase-1 (192). Similar observations have been made in SLE, where anti-dsDNA and anti-histone antibodies are thought to protect NETs against degradation by circulating DNase (193).
Mechanistically, after an infection, the germinal center B-cell response is crucial for the development of high-affinity antibodies through the processes of class-switching, somatic hypermutation, and affinity maturation. Impaired germinal center B-cell responses, as observed in severe and critical COVID-19 cases, lead to the emergence of extrafollicular B-cell responses (194, 195). This aberrant activation pathway induces the production of polyreactive autoantibodies with limited somatic hypermutation, similar to patterns observed in SLE (196). These polyreactive antibodies, along with an abundant supply of self-antigens from dead or dying cells and NETs, may drive the development of autoantibodies in COVID-19 (197).
In the context of LC, COVID-19 survivors exhibit higher detectable levels of ANAs 3 months and 12 months post-infection compared to age- and sex-matched healthy controls (198). Persistently positive ANA titers are associated with LC symptoms of fatigue, dyspnea, and cough (198). ANA positivity also correlates with higher levels of TNF-α (198). The presence of autoantibodies associated with antiphospholipid syndrome, including anti-cardiolipin, anti-phosphatidylserine/prothrombin, and anti- β2 glycoprotein, is evident in approximately half of hospitalized COVID-19 patients (199). Moreover, these IgG autoantibodies induce NETosis in neutrophils isolated from healthy patients and accelerate venous thrombosis when injected into mice (199). More recently, it was demonstrated that a significant fraction of LC patients exhibit positivity for IgM/IgG anti-cardiolipin and anti-β2 glycoprotein autoantibodies (186). In the same cohort, LC patients demonstrated higher levels of NET markers than healthy controls, indicating incomplete resolution of NETs after recovery from SARS-CoV-2 (186).
These findings draw intriguing parallels between COVID-19 and autoimmune diseases like SLE, highlighting the presence of NETs as a potential source of neoantigens. Dysregulated humoral responses, coupled with the sustained presence of NETs, contribute to the initiation and perpetuation of autoimmunity. However, discerning the pathogenicity of specific autoantibodies in LC remains largely unchartered territory, as existing data does not establish causal relationships.
5.3. NETs in long COVID lung disease
Respiratory symptoms such as cough and dyspnea are frequently observed in individuals with LC (200, 201). A meta-analysis of over 257,000 COVID-19 patients reported that dyspnea persisted for > 12 months after the initial COVID-19 infection in 31% of cases (202). Radiologically, a combination of persistent inflammation (ground glass opacities and consolidation) and fibrosis (fibrotic bands, interlobular septal thickening, and honeycombing) can be observed (203, 204). Persistent inflammation dominates the early post-acute phase, with fibrosis becoming more prevalent during the follow-up period (203). Significantly, approximately 45% of severe COVID-19 survivors will develop pulmonary fibrosis (205). Risk factors for the development of pulmonary fibrosis as LC include various indicators of more severe disease, such as ICU admission, mechanical ventilation, longer hospitalization, and steroid/immunoglobulin treatment (205).
One study by George et al. study has provided substantial insight into the role of NETs in the pathogenesis of LC lung disease, particularly by including a comparison group of patients who experienced complete clinical and radiologic resolution of acute COVID-19 symptoms (206). The authors demonstrated that a subset of severe COVID-19 survivors developed interstitial lung disease (ILD)-related changes on chest computed tomography scan at 3-6 months post-SARS-CoV-2 infection, accompanied by a restrictive pattern on pulmonary function testing. Patients with persistent interstitial changes exhibited significantly elevated neutrophil counts and serum MPO concentrations compared to controls, which positively correlated with the radiologic extent of pulmonary disease. Comparing the plasma proteome of these patients revealed that the neutrophil chemoattractant IL-17 was the only protein significantly associated with persistent ILD on multivariate analysis (OR 3.72, 95% CI 1.20-16.84, p=0.0403) (206). Furthermore, neutrophil chemokines CXCL1 and CXCL8 positively correlated with the degree of restrictive disease on pulmonary function testing, while CXCL8 and the inflammasome-related cytokine IL-18, along with its receptor IL-18R1, were directly associated with the radiological extent of interstitial disease (206). These differences were also reflected in nasal brushing samples taken to model mucosal immunological changes in the upper airways. In vitro experiments using alveolar epithelial cell lines showed that purified NETs increased the expression of fibronectin-1, vascular endothelial growth factor, and alpha-smooth muscle actin, while reducing E-cadherin expression, indicating that NETs drive epithelial-to-mesenchymal transition (EMT) and subsequent extracellular matrix deposition, which are established processes in pulmonary fibrosis (206). These changes were primarily driven by the host response to infection, such as through NETs, rather than direct viral infection of alveolar epithelial cells (206).
These findings are consistent with lung autopsies of deceased COVID-19 patients revealing the presence of alveolar epithelial cells co-expressing mesenchymal markers (207). In vitro experiments demonstrate that co-culturing alveolar epithelial cells with neutrophils, alveolar macrophages, and SARS-CoV-2 virus results in the production of factors such as TGF-β, IL-8, and IL-1β by alveolar macrophages, along with NET production, ultimately leading to a complete EMT signature. Notably, removing either neutrophils or alveolar macrophages resulted in an incomplete EMT phenotype (207). These findings support the notion of an alveolar macrophage/neutrophil/NETosis axis, whereby factors released by alveolar macrophage-derived factors induce NETosis, which, in turn, promotes EMT in pneumocytes.
Another study explored the role of Kruppel-like factor 2 (KLF2) in pulmonary sequelae of LC (208). KLF2 is a fibroblast protein, and its downregulation has been implicated in fibrosing disorders (209, 210). Lung fibroblasts stimulated with plasma from severe COVID-19 patients downregulate KLF2 and acquire a pre-fibrotic phenotype (208). Treating lung fibroblasts with a combination of DNAse-1 (to degrade NETs) and JAK/IL-6 inhibitors baritinib/tocilizumab (to attenuate inflammation) normalized KLF2 expression. Significantly, COVID-19 patients treated with this combination showed better outcomes compared to those receiving standard-of-care therapy. Furthermore, exposing lung fibroblasts to the plasma of treated patients resulted in higher KLF2 expression (208).
Together, these findings suggest that NETs and the inflammatory environment in the circulation and lung parenchyma of COVID-19 patients, particularly in severe cases, induce fibrotic phenotypes in alveolar epithelial cells and lung fibroblasts, which may explain the development of pulmonary fibrosis observed in a significant proportion of severe COVID-19 survivors.
5.4. NETs in the cardiovascular manifestations of long COVID
Several prospective and retrospective studies have consistently demonstrated a higher incidence of vascular pathologies, such as arterial thrombosis, venous thrombosis, atherosclerosis, vasculitis, and hypertension, in patients with LC (211–214). Notably, a prospective study of 153,760 COVID-19 patients revealed that convalescent individuals had a significantly higher risk of developing future cardiovascular disease, cerebrovascular disease, thromboembolic events, and ischemic heart disease compared to healthy contemporary and historical controls (211). This highlights a distinctive vascular feature of LC, characterized by widespread activation of pro-coagulant pathways (215–218). Indeed, elevated levels of pro-inflammatory and pro-thrombotic mediators have been observed in LC patients compared to healthy controls (219). It has recently been shown that NETosis persists at a greater level in LC patients compared to convalescent recovering patients (184). Persistent activation of pathways related to immunothrombosis and neutrophil activation has also been observed in COVID-19 survivors 6 months after the initial SARS-CoV-2 infection (220).
NETs are critical mediators of immunothrombosis and endotheliitis, exerting their function through various mechanisms (221, 222). NETs have also been implicated in the development of atherosclerosis, where endothelial cells promote NET formation. In turn, NETs lead to an increase in pro-inflammatory signaling and subsequent recruitment of immune cells to atherosclerotic plaques (223–228). NETs have also been shown to participate in the pathophysiology of ANCA-associated vasculitides (229), suggesting a potential link between autoimmunity, NETs, and vasculopathy. Promising results have been observed with NET-targeted therapies in the treatment of vascular pathologies, further supporting the involvement of NETs (230–233). Additionally, a larger burden of NETs has been observed in the coronary thrombi of COVID-19 convalescent patients with ST-elevation myocardial infarction when compared to historical controls (12, 234). These findings provide evidence of plausible evidence of NETs in the pathogenesis of vascular disorders in LC.
Cardiac involvement is an archetypal feature of LC and encompasses a wide range of presentations, including cardiac inflammation, cardiac fibrosis, dysrhythmias, ischemic heart disease, and cardiac impairment (235, 236). Cohort studies utilizing cardiac magnetic resonance imaging in patients with LC have demonstrated evidence of impaired ventricular function in addition to increased cardiac edema and inflammation (237–239). Additionally, patients with LC demonstrated a heightened incidence of myocardial injury particularly in the form of myocardial fibrosis (240). The interplay between NETs and cardiac disorders in several viral and bacterial infections was recently reviewed (241). Clinical evidence for the involvement of NETs in COVID-19-induced cardiac inflammation has recently been established through autopsy reports from 21 SARS-CoV-2 infected individuals, showing the presence of NETs in all patients and its association with myocarditis and cardiac injury (242). Additionally, targeting NETs in a mouse model of COVID-19 through DNase I therapy resulted in the attenuation of cardiac injury (243). Neutrophils recruited to the heart via the cytokine midkine (MK), which then induces NETosis, contribute to the pathogenesis of myocarditis and cardiac inflammation (244). Targeting MK attenuates neutrophil infiltration and NET formation, associated with a reduction in ventricular systolic dysfunction and myocardial fibrosis (244). In vitro studies have highlighted a potential role for NETs in the development of cardiac fibrosis through enhancing fibroblast migration and promoting cardiac myofibroblast differentiation (245). These findings suggest a potential role for NETs in non-ischemic cardiac injury in LC.
5.5. NETs in neurologic manifestations of long COVID
Another prominent feature of LC is the array of neurological complications the sizeable chronic burden of which has been indicated by numerous studies (246–249). On a gross scale, a UK BioBank study analyzed brain MRI scans pre- and post-infection and observed a greater clinical burden of cognitive decline and radiological changes of reduction in brain size and gray matter atrophy affecting the hippocampus and orbitofrontal cortex in the post-infection group (250).
Mechanistically, direct viral invasion of the brain does not appear to be prominent except in very severe acute COVID-19 cases (7). Instead, neuroinflammation, micro-clots, and the activation of CNS-resident glial cells are hypothesized to be crucial mediators of LC-associated neurological sequelae (251). Experiments in ACE2-transgenic mice have indicated that even a mild SARS-CoV-2 respiratory infection raises systemic cytokine levels, such as CCL11, which can be neurotoxic by inducing reactive states in microglia (252). Indicators of glial cell reactivity are elevated amongst LC patients with persistent depressive symptoms (253). A study of 76 LC patients experiencing “brain fog” (encompassing headache, fatigue, malaise, and altered level of consciousness) was the first to objectively demonstrate COVID-19-associated BBB disruption by utilizing neurological biomarkers and dynamic contrast-enhanced magnetic resonance imaging (254). Sustained elevations in S100β, IL-8, TGF-β, and GFAP were observed in brain fog LC patients, indicating persistent inflammation. Importantly, the adhesion of peripheral blood mononuclear cells to brain microvascular endothelial cells was enhanced in patients with brain fog, and exposing endothelial cells to the serum of these patients triggered endothelial cell activation (254).
A distinctive feature of LC is the higher propensity for developing ischemic strokes (251). Lee et al. documented the presence of microvascular injury indicated by scattered microthrombi, endothelial activation associated with the adhesion of autoantibodies and complement proteins, and BBB disruption indicated by the perivascular presence of fibrinogen in brain autopsies of individuals who died suddenly with or after COVID-19 (255, 256). Co-localizing with fibrinogen were microglia/macrophages, CD8+ T-cells, and reactive astrocytes, with areas of neuronal loss observed from microglia phagocytosis (255, 256). Fibrin clots are also generally elevated in the blood of LC patients (216). A recent study associated elevated serum fibrinogen and D-dimer levels relative to C-reactive protein during acute admission with neurocognitive deficits 6 and 12 months after acute COVID-19, supporting the notion of an acute inflammatory response being responsible for the long-lasting neurological effects of LC (257). Together, these findings suggest that BBB disruption during acute COVID-19 leads to the spillover of fibrinogen which, along with propagating a hypercoagulable state and micro-clots (258), foster neurotoxic resident glial cell phenotypes that damage neurons in a subtle but debilitating manner with long-lasting neurocognitive consequences (see (259) for a detailed review on the neurological effects of fibrinogen).
The brain is typically devoid of neutrophils and NETs due to the integrity of the BBB (260). However, in scenarios where the BBB is compromised, neutrophils infiltrate and NETs are often visualized in brain tissue where they contribute towards ongoing neuroinflammation (260, 261). Recent studies proposed that COVID-19 facilitates BBB disruption by increasing the expression of matrix metalloproteinase-9 (MMP-9) which leads to basement membrane degradation (262, 263). The levels of NETs in the brain have been directly related to the degree of neuroinflammation in model organisms of Alzheimer’s disease, meningitis, ischemic stroke, and traumatic brain injury (264). That being said, direct evidence of the involvement of NETs in cerebral micro-clots and neuroinflammation in acute and LC is lacking. The fact remains that many of the autoantibodies (82), complement proteins (121), fibrinogen (265), Von-Willebrand factor (266), and platelets (267) that are hypothesized to be key to the micro-clot formation in LC and driving its neurological manifestations are known to be intertwined with neutrophil biology and NET production. Hence, directing research efforts toward identifying and tackling NETs may improve our understanding of the neurological sequelae of LC.
5.6. Cellular senescence and NETosis
Cellular senescence refers to a state of cell cycle arrest accompanied by the release of inflammatory molecules collectively termed the senescence-associated secretory phenotype (SASP) (268). Cellular senescence is associated with a chronic low-grade inflammatory state and is causally implicated in aging and various chronic diseases, including pulmonary fibrosis, neurodegeneration, and cardiovascular disease (269–277). Viral infections, including SARS-CoV-2, trigger a cellular stress response, culminating in the induction of senescence, termed virus-induced senescence (VIS) (278).
The intracellular signaling pathways mediating SARS-CoV-2—induced VIS have been extensively reviewed elsewhere (278). Suffice it to say that multiple studies have shown elevated levels of senescence markers in the upper and lower respiratory tract of COVID-19 patients, indicative of SARS-CoV-2 infection-related VIS, which results in the elaboration of a pro-inflammatory SASP that recruits and induces pro-inflammatory M1 phenotypes in macrophages (279–282). Notably, some samples with a high burden of senescent cells did not show detectable viral infection, suggesting that senescence can persist even after clearance of SARS-CoV-2 (278, 280). Linking senescence induction to COVID-19 pathology, Lee et al. demonstrated that supernatant from SARS-CoV-2-induced VIS cells can induce endothelial cell senescence or apoptosis, promote M1 macrophage polarization, activate platelets, trigger NET production, and accelerate thrombosis (280). Treating with senolytic drugs, which eliminate senescent cells by causing their selective apoptosis, reduced the levels of SASP-reminiscent pro-inflammatory cytokines in hamster models of SARS-CoV-2 infection. However, the histopathological impact on inflammation and thrombosis did not significantly improve with senolytic treatment (280).
In the context of LC, it is important to note that senescent cells are typically cleared by the immune system because of their chemoattractive SASP (283). However, in certain circumstances, such as tumorigenesis, senescent cells can persist and contribute to long-term disease recurrence by evading immune surveillance (284–286). Although data on whether senescent cells persist and drive LC phenotypes are currently lacking, ongoing studies have demonstrated SARS-CoV-2 infection-induced VIS of human brain organoids in corticothalamic neurons and GABAergic ganglionic eminence neurons, which are responsible for modulating neuronal circuitry and processing of sensory information (287). SARS-CoV-2 was shown to induce the loss of dopaminergic neurons in the brainstem responsible for coordination and consciousness, potentially explaining abnormalities in these processes in LC (287). However, multiple studies have suggested that direct SARS-CoV-2 infection of the brain likely plays an insignificant role in acute and long neuro-COVID (251).
Additionally, the downstream consequences of senescence induction and pro-inflammatory SASP elaboration—in terms of changes in neuronal function, synaptic plasticity, astrocyte or microglial activation, or the recruitment of circulating innate and adaptive immune cells—are currently unclear. The BBB disruption in LC does support the role of neutrophils and NETs, as is indicated by their role in other CNS pathologies that involve BBB disruption (261). Other than the data above, no studies directly observing senescent cell persistence and their roles in LC in other organ systems such as the lungs have not yet been conducted, but several hypotheses have been put forward (288, 289). Exploring the link between senescence and NETosis is particularly important given the rapid evolution of senolytic therapies into clinical trials and the feasibility of targeting senescent cells; such research may uncover therapeutic targets with great potential for translation into clinical applications.
6. Conclusions and outlook
The findings discussed provided valuable insights into the involvement of neutrophils and NETs in LC. However, there are still unanswered questions that, once addressed, could position NETs as viable targets for therapeutic interventions. Understanding the heterogeneity of neutrophils in LC, exploring the factors that sustain increased NETosis induction levels after SARS-CoV-2 resolution, and examining the impact of NETs on other organ systems, particularly the cardiovascular and nervous systems, are crucial areas to investigate. It is somewhat counterintuitive that short-lived neutrophils can exhibit a sustained activation response in LC, perhaps being influenced by alterations in effector phenotypes of long-lived cells or microenvironmental changes in the bone marrow (290). The impact of location must also be explored, i.e., considering compartmentalized/organ-specific pathophysiological differences, influenced perhaps by the potential role of persistent senescent cells and their influence on immune cell recruitment, is vital. Identifying factors—such as autoantibodies—that determine NET degradation capacity in the post-acute phase, as well as elucidating the specific NET components responsible for organ-specific pathology such as lung fibrosis, will enhance our understanding of LC. Addressing these uncertainties may pave the way for clinically applicable strategies aimed at targeting NETs, potentially alleviating this emerging health crisis.
Author contributions
AS: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. MO: Writing – original draft, Writing – review & editing. IA: Writing – original draft. GA: Writing – original draft. HA: Writing – original draft. SA: Writing – original draft. BS: Writing – original draft. KA: Writing – review & editing. AY: Conceptualization, Writing – review & editing.
Acknowledgments
Figures were created with Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med (2022) 28(7):1461–7. doi: 10.1038/s41591-022-01840-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ballering AV, van Zon SKR, Hartman TC, Rosmalen JGM. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet (2022) 400(10350):452–61. doi: 10.1016/S0140-6736(22)01214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun (2022) 101:93–135. doi: 10.1016/j.bbi.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malesevic S, Sievi NA, Baumgartner P, Roser K, Sommer G, Schmidt D, et al. Impaired health-related quality of life in long-COVID syndrome after mild to moderate COVID-19. Sci Rep (2023) 13(1):7717. doi: 10.1038/s41598-023-34678-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fawzy NA, Abou Shaar B, Taha RM, Arabi TZ, Sabbah BN, Alkodaymi MS, et al. A systematic review of trials currently investigating therapeutic modalities for post-acute COVID-19 syndrome and registered on WHO International Clinical Trials Platform. Clin Microbiol Infect (2023) 29(5):570–7. doi: 10.1016/j.cmi.2023.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol (2023) 21(3):133–46. doi: 10.1038/s41579-022-00846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altmann DM, Whettlock EM, Liu S, Arachchillage DJ, Boyton RJ. The immunology of long COVID. Nat Rev Immunol (2023). doi: 10.1038/s41577-023-00904-7 [DOI] [PubMed] [Google Scholar]
- 8. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol (2018) 18(2):134–47. doi: 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 9. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol (2011) 11(8):519–31. doi: 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- 10. Shafqat A, Shafqat S, Salameh SA, Kashir J, Alkattan K, Yaqinuddin A. Mechanistic insights into the immune pathophysiology of COVID-19; an in-depth review. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.835104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alkattan W, Yaqinuddin A, Shafqat A, Kashir J. NET-mediated pathogenesis of COVID-19: the role of NETs in hepatic manifestations. J Health Allied Sci NU (2022). doi: 10.1055/s-0041-1741418 [DOI] [Google Scholar]
- 12. Farouk AF, Shafqat A, Shafqat S, Kashir J, Alkattan K, Yaqinuddin A. COVID-19 associated cardiac disease: Is there a role of neutrophil extracellular traps in pathogenesis? AIMS Mol Science (2021) 8(4):275–90. doi: 10.3934/molsci.2021021 [DOI] [Google Scholar]
- 13. Al-Kuraishy HM, Al-Gareeb AI, Al-Hussaniy HA, Al-Harcan NAH, Alexiou A, Batiha GE. Neutrophil Extracellular Traps (NETs) and Covid-19: A new frontiers for therapeutic modality. Int Immunopharmacol (2022) 104:108516. doi: 10.1016/j.intimp.2021.108516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Apel F, Zychlinsky A, Kenny EF. The role of neutrophil extracellular traps in rheumatic diseases. Nat Rev Rheumatol (2018) 14(8):467–75. doi: 10.1038/s41584-018-0039-z [DOI] [PubMed] [Google Scholar]
- 15. Bonaventura A, Vecchié A, Abbate A, Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells (2020) 9(1):231. doi: 10.3390/cells9010231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klopf J, Brostjan C, Eilenberg W, Neumayer C. Neutrophil extracellular traps and their implications in cardiovascular and inflammatory disease. Int J Mol Sci (2021) 22(2):559. doi: 10.3390/ijms22020559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303(5663):1532–5. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 18. Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J, et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PloS One (2012) 7(10):e48111. doi: 10.1371/journal.pone.0048111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sofoluwe A, Bacchetta M, Badaoui M, Kwak BR, Chanson M. ATP amplifies NADPH-dependent and-independent neutrophil extracellular trap formation. Sci Rep (2019) 9(1):1–11. doi: 10.1038/s41598-019-53058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern–activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology (2015) 62(2):600–14. doi: 10.1002/hep.27841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang YM, Wang H, Wang C, Chen M, Zhao MH. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol (2015) 67(10):2780–90. doi: 10.1002/art.39239 [DOI] [PubMed] [Google Scholar]
- 22. Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med (2007) 13(4):463–9. doi: 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- 23. Jiao Y, Li W, Wang W, Tong X, Xia R, Fan J, et al. Platelet-derived exosomes promote neutrophil extracellular trap formation during septic shock. Crit Care (2020) 24(1):380. doi: 10.1186/s13054-020-03082-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer L, Damgaard C, Holmstrup P, Nielsen C. Influence of complement on neutrophil extracellular trap release induced by bacteria. J periodontal Res (2016) 51(1):70–6. doi: 10.1111/jre.12284 [DOI] [PubMed] [Google Scholar]
- 25. de Bont CM, Boelens WC, Pruijn GJM. NETosis, complement, and coagulation: a triangular relationship. Cell Mol Immunol (2019) 16(1):19–27. doi: 10.1038/s41423-018-0024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wigerblad G, Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol (2022) 23(5):274–88. doi: 10.1038/s41577-022-00787-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med (2016) 22(2):146–53. doi: 10.1038/nm.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon H-U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differentiation (2009) 16(11):1438–44. doi: 10.1038/cdd.2009.96 [DOI] [PubMed] [Google Scholar]
- 29. Biermann MH, Podolska MJ, Knopf J, Reinwald C, Weidner D, Maueröder C, et al. Oxidative burst-dependent NETosis is implicated in the resolution of necrosis-associated sterile inflammation. Front Immunol (2016) 7:557. doi: 10.3389/fimmu.2016.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol (2010) 191(3):677–91. doi: 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci (2015) 112(9):2817–22. doi: 10.1073/pnas.1414055112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amulic B, Knackstedt SL, Abu Abed U, Deigendesch N, Harbort CJ, Caffrey BE, et al. Cell-cycle proteins control production of neutrophil extracellular traps. Dev Cell (2017) 43(4):449–62.e5. doi: 10.1016/j.devcel.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 33. Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol (2018) 3(26):eaar6689. doi: 10.1126/sciimmunol.aar6689 [DOI] [PubMed] [Google Scholar]
- 34. Spel L, Martinon F. Gasdermin D opens the way for NETs. Nat Rev Rheumatol (2018) 14(12):690–2. doi: 10.1038/s41584-018-0124-3 [DOI] [PubMed] [Google Scholar]
- 35. Rohrbach A, Slade D, Thompson P, Mowen K. Activation of PAD4 in NET formation. Front Immunol (2012) 3. doi: 10.3389/fimmu.2012.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol (2018) 3(26):eaar6676. doi: 10.1126/sciimmunol.aar6676 [DOI] [PubMed] [Google Scholar]
- 37. Stojkov D, Claus MJ, Kozlowski E, Oberson K, Schären OP, Benarafa C, et al. NET formation is independent of gasdermin D and pyroptotic cell death. Sci Signaling (2023) 16(769):eabm0517. doi: 10.1126/scisignal.abm0517 [DOI] [PubMed] [Google Scholar]
- 38. Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, Thompson PR, et al. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife (2017) 6:e24437. doi: 10.7554/eLife.24437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guiducci E, Lemberg C, Küng N, Schraner E, Theocharides APA, LeibundGut-Landmann S. Candida albicans-induced NETosis is independent of peptidylarginine deiminase 4. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.01573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discovery. (2020) 19(4):253–75. doi: 10.1038/s41573-019-0054-z [DOI] [PubMed] [Google Scholar]
- 41. Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. J Clin Invest (1982) 70(3):550–7. doi: 10.1172/JCI110647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rodríguez-Espinosa O, Rojas-Espinosa O, Moreno-Altamirano MM, López-Villegas EO, Sánchez-García FJ. Metabolic requirements for neutrophil extracellular traps formation. Immunology (2015) 145(2):213–24. doi: 10.1111/imm.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amini P, Stojkov D, Felser A, Jackson CB, Courage C, Schaller A, et al. Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat Commun (2018) 9(1):2958. doi: 10.1038/s41467-018-05387-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Awasthi D, Nagarkoti S, Sadaf S, Chandra T, Kumar S, Dikshit M. Glycolysis dependent lactate formation in neutrophils: A metabolic link between NOX-dependent and independent NETosis. Biochim Biophys Acta (BBA) - Mol Basis Dis (2019) 1865(12):165542. doi: 10.1016/j.bbadis.2019.165542 [DOI] [PubMed] [Google Scholar]
- 45. Behnen M, Möller S, Brozek A, Klinger M, Laskay T. Extracellular acidification inhibits the ROS-dependent formation of neutrophil extracellular traps. Front Immunol (2017) 8:184. doi: 10.3389/fimmu.2017.00184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol (2018) 55(3):379–90. doi: 10.1007/s12016-018-8702-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jayne DRW, Bruchfeld AN, Harper L, Schaier M, Venning MC, Hamilton P, et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol (2017) 28(9):2756–67. doi: 10.1681/ASN.2016111179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iwata K, Doi A, Ohji G, Oka H, Oba Y, Takimoto K, et al. Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Intern Med (2010) 49(22):2423–32. doi: 10.2169/internalmedicine.49.4010 [DOI] [PubMed] [Google Scholar]
- 49. Biron BM, Chung CS, O'Brien XM, Chen Y, Reichner JS, Ayala A. Cl-amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J Innate Immun (2017) 9(1):22–32. doi: 10.1159/000448808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao T, Pan B, Alam HB, Liu B, Bronson RT, Deng Q, et al. Protective effect of Cl-amidine against CLP-induced lethal septic shock in mice. Sci Rep (2016) 6:36696. doi: 10.1038/srep36696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fadini GP, Menegazzo L, Rigato M, Scattolini V, Poncina N, Bruttocao A, et al. NETosis delays diabetic wound healing in mice and humans. Diabetes (2016) 65(4):1061–71. doi: 10.2337/db15-0863 [DOI] [PubMed] [Google Scholar]
- 52. Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci (1990) 87(23):9188–92. doi: 10.1073/pnas.87.23.9188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holliday ZM, Earhart AP, Alnijoumi MM, Krvavac A, Allen LH, Schrum AG. Non-randomized trial of dornase alfa for acute respiratory distress syndrome secondary to covid-19. Front Immunol (2021) 12:714833. doi: 10.3389/fimmu.2021.714833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weber AG, Chau AS, Egeblad M, Barnes BJ, Janowitz T. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: a case series. Mol Med (2020) 26(1):91. doi: 10.1186/s10020-020-00215-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fisher J, Mohanty T, Karlsson CAQ, Khademi SMH, Malmström E, Frigyesi A, et al. Proteome profiling of recombinant DNase therapy in reducing NETs and aiding recovery in COVID-19 patients. Mol Cell Proteomics (2021) 20:100113. doi: 10.1016/j.mcpro.2021.100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol (2020) 21(7):736–45. doi: 10.1038/s41590-020-0669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Adrover JM, Carrau L, Daßler-Plenker J, Bram Y, Chandar V, Houghton S, et al. Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection. JCI Insight (2022) 7(5):e157342. doi: 10.1172/jci.insight.157342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ali RA, Gandhi AA, Meng H, Yalavarthi S, Vreede AP, Estes SK, et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun (2019) 10(1):1916. doi: 10.1038/s41467-019-09801-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eby JC, Gray MC, Hewlett EL. Cyclic AMP-mediated suppression of neutrophil extracellular trap formation and apoptosis by the Bordetella pertussis adenylate cyclase toxin. Infect Immun (2014) 82(12):5256–69. doi: 10.1128/IAI.02487-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shishikura K, Horiuchi T, Sakata N, Trinh DA, Shirakawa R, Kimura T, et al. Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. Br J Pharmacol (2016) 173(2):319–31. doi: 10.1111/bph.13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol (2021) 19(3):155–70. doi: 10.1038/s41579-020-00468-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell (2020) 181(2):271–80.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med (2020) 26(5):681–7. doi: 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, 3rd, et al. SARS-coV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell (2020) 182(2):429–46.e14. doi: 10.1016/j.cell.2020.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Arya R, Kumari S, Pandey B, Mistry H, Bihani SC, Das A, et al. Structural insights into SARS-CoV-2 proteins. J Mol Biol (2021) 433(2):166725. doi: 10.1016/j.jmb.2020.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ziegler CG, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell (2020) 181(5):1016–35.e19. doi: 10.1016/j.cell.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chu H, Chan JF, Wang Y, Yuen TT, Chai Y, Hou Y, et al. Comparative replication and immune activation profiles of SARS-coV-2 and SARS-coV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis (2020) 71(6):1400–9. doi: 10.1093/cid/ciaa410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grant RA, Morales-Nebreda L, Markov NS, Swaminathan S, Querrey M, Guzman ER, et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature (2021) 590(7847):635–41. doi: 10.1038/s41586-020-03148-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Paludan SR, Mogensen TH. Innate immunological pathways in COVID-19 pathogenesis. Sci Immunol (2022) 7(67):eabm5505. doi: 10.1126/sciimmunol.abm5505 [DOI] [PubMed] [Google Scholar]
- 70. Diamond MS, Kanneganti T-D. Innate immunity: the first line of defense against SARS-CoV-2. Nat Immunol (2022) 23(2):165–76. doi: 10.1038/s41590-021-01091-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Minkoff JM, tenOever B. Innate immune evasion strategies of SARS-CoV-2. Nat Rev Microbiol (2023) 21(3):178–94. doi: 10.1038/s41579-022-00839-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lowery SA, Sariol A, Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell Host Microbe (2021) 29(7):1052–62. doi: 10.1016/j.chom.2021.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shafqat A, Arabi TZ, Sabbah BN, Abdulkader HS, Shafqat S, Razak A, et al. Understanding COVID-19 vaccines today: are T-cells key players? Vaccines (Basel) (2022) 10(6):904. doi: 10.3390/vaccines10060904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shafqat A, Omer MH, Ahmad O, Niaz M, Abdulkader HS, Shafqat S, et al. SARS-CoV-2 epitopes inform future vaccination strategies. Front Immunol (2022) 13. doi: 10.3389/fimmu.2022.1041185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sette A, Crotty S. Adaptive immunity to SARS-coV-2 and COVID-19. Cell (2021) 184(4):861–80. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. The Novel Coronavirus Pneumonia Emergency Response Epidemiology T . The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) - China, 2020. China CDC Wkly (2020) 2(8):113–22. doi: 10.46234/ccdcw2020.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ulloque-Badaracco JR, Ivan Salas-Tello W, Al-kassab-Córdova A, Alarcón-Braga EA, Benites-Zapata VA, Maguiña JL, et al. Prognostic value of neutrophil-to-lymphocyte ratio in COVID-19 patients: a systematic review and meta-analysis. Int J Clin Practice. (2021) 75(11):e14596. doi: 10.1111/ijcp.14596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Panda R, Castanheira FVS, Schlechte JM, Surewaard BGJ, Shim HB, Zucoloto AZ, et al. A functionally distinct neutrophil landscape in severe COVID-19 reveals opportunities for adjunctive therapies. JCI Insight (2022) 7(2):e152291. doi: 10.1172/jci.insight.152291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Middleton EA, He X-Y, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood (2020) 136(10):1169–79. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xie X, Shi Q, Wu P, Zhang X, Kambara H, Su J, et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol (2020) 21(9):1119–33. doi: 10.1038/s41590-020-0736-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Grieshaber-Bouyer R, Radtke FA, Cunin P, Stifano G, Levescot A, Vijaykumar B, et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat Commun (2021) 12(1):2856. doi: 10.1038/s41467-021-22973-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wigerblad G, Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol (2023) 23(5):274–88. doi: 10.1038/s41577-022-00787-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Deniset JF, Kubes P. Neutrophil heterogeneity: Bona fide subsets or polarization states? J Leukoc Biol (2018) 103(5):829–38. doi: 10.1002/JLB.3RI0917-361R [DOI] [PubMed] [Google Scholar]
- 84. Carlucci PM, Purmalek MM, Dey AK, Temesgen-Oyelakin Y, Sakhardande S, Joshi AA, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight (2018) 3(8):e99276. doi: 10.1172/jci.insight.99276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol (2010) 184(6):3284–97. doi: 10.4049/jimmunol.0902199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Silvin A, Chapuis N, Dunsmore G, Goubet A-G, Dubuisson A, Derosa L, et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell (2020) 182(6):1401–18.e18. doi: 10.1016/j.cell.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schulte-Schrepping J, Reusch N, Paclik D, Baßler K, Schlickeiser S, Zhang B, et al. Severe COVID-19 is marked by a dysregulated myeloid cell compartment. Cell (2020) 182(6):1419–40.e23. doi: 10.1016/j.cell.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wu P, Chen D, Ding W, Wu P, Hou H, Bai Y, et al. The trans-omics landscape of COVID-19. Nat Commun (2021) 12(1):4543. doi: 10.1038/s41467-021-24482-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Morrissey SM, Geller AE, Hu X, Tieri D, Ding C, Klaes CK, et al. A specific low-density neutrophil population correlates with hypercoagulation and disease severity in hospitalized COVID-19 patients. JCI Insight (2021) 6(9):e148435. doi: 10.1172/jci.insight.148435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sacchi A, Grassi G, Bordoni V, Lorenzini P, Cimini E, Casetti R, et al. Early expansion of myeloid-derived suppressor cells inhibits SARS-CoV-2 specific T-cell response and may predict fatal COVID-19 outcome. Cell Death Disease. (2020) 11(10):921. doi: 10.1038/s41419-020-03125-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Burnett CE, Okholm TLH, Tenvooren I, Marquez DM, Tamaki S, Munoz Sandoval P, et al. Mass cytometry reveals a conserved immune trajectory of recovery in hospitalized COVID-19 patients. Immunity (2022) 55(7):1284–98.e3. doi: 10.1101/2022.03.15.484467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sinha S, Rosin NL, Arora R, Labit E, Jaffer A, Cao L, et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19. Nat Med (2022) 28(1):201–11. doi: 10.1038/s41591-021-01576-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Combes AJ, Courau T, Kuhn NF, Hu KH, Ray A, Chen WS, et al. Global absence and targeting of protective immune states in severe COVID-19. Nature (2021) 591(7848):124–30. doi: 10.1038/s41586-021-03234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Castanheira FVS, Kubes P. Neutrophils during SARS-CoV-2 infection: Friend or foe? Immunol Rev (2023) 314(1):399–412. doi: 10.1111/imr.13175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Reusch N, De Domenico E, Bonaguro L, Schulte-Schrepping J, Baßler K, Schultze JL, et al. Neutrophils in COVID-19. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.652470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Voiriot G, Dorgham K, Bachelot G, Fajac A, Morand-Joubert L, Parizot C, et al. Identification of bronchoalveolar and blood immune-inflammatory biomarker signature associated with poor 28-day outcome in critically ill COVID-19 patients. Sci Rep (2022) 12(1):9502. doi: 10.1038/s41598-022-13179-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jorens PG, Van Damme J, De Backer W, Bossaert L, De Jongh RF, Herman AG, et al. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine (1992) 4(6):592–7. doi: 10.1016/1043-4666(92)90025-M [DOI] [PubMed] [Google Scholar]
- 98. Ronit A, Berg RMG, Bay JT, Haugaard AK, Ahlström MG, Burgdorf KS, et al. Compartmental immunophenotyping in COVID-19 ARDS: A case series. J Allergy Clin Immunol (2021) 147(1):81–91. doi: 10.1016/j.jaci.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med (2020) 26(6):842–4. doi: 10.1038/s41591-020-0901-9 [DOI] [PubMed] [Google Scholar]
- 100. Eddins DJ, Yang J, Kosters A, Giacalone VD, Pechuan-Jorge X, Chandler JD, et al. Transcriptional reprogramming of infiltrating neutrophils drives lung pathology in severe COVID-19 despite low viral load. Blood Adv (2023) 7(5):778–99. doi: 10.1182/bloodadvances.2022008834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wauters E, Van Mol P, Garg AD, Jansen S, Van Herck Y, Vanderbeke L, et al. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res (2021) 31(3):272–90. doi: 10.1038/s41422-020-00455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med (2020) 217(12):e20201129. doi: 10.1101/2020.06.08.20125823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wu M, Chen Y, Xia H, Wang C, Tan CY, Cai X, et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc Natl Acad Sci USA (2020) 117(45):28336–43. doi: 10.1073/pnas.2018030117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Guo Q, Zhao Y, Li J, Liu J, Yang X, Guo X, et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe (2021) 29(2):222–35.e4. doi: 10.1016/j.chom.2020.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med (2020) 217(6):e20200652. doi: 10.1084/jem.20200652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Thierry AR, Roch B. SARS-CoV2 may evade innate immune response, causing uncontrolled neutrophil extracellular traps formation and multi-organ failure. Clin Sci (Lond) (2020) 134(12):1295–300. doi: 10.1042/CS20200531 [DOI] [PubMed] [Google Scholar]
- 107. Tomar B, Anders HJ, Desai J, Mulay SR. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells (2020) 9(6):1383. doi: 10.3390/cells9061383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight (2020) 5(11):e138999. doi: 10.1172/jci.insight.138999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Herrera VLM, Bosch NA, Lok JJ, Nguyen MQ, Lenae KA, deKay JT, et al. Circulating neutrophil extracellular trap (NET)-forming ‘rogue’ neutrophil subset, immunotype [DEspR + CD11b +], mediate multi-organ failure in COVID-19—an observational study. Trans Med Commun (2023) 8(1):12. doi: 10.1186/s41231-023-00143-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liana P, Liberty IA, Murti K, Hafy Z, Salim EM, Zulkarnain M, et al. A systematic review on neutrophil extracellular traps and its prognostication role in COVID-19 patients. Immunologic Res (2022) 70(4):449–60. doi: 10.1007/s12026-022-09293-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ackermann M, Kamp JC, Werlein C, Walsh CL, Stark H, Prade V, et al. The fatal trajectory of pulmonary COVID-19 is driven by lobular ischemia and fibrotic remodelling. EBioMedicine (2022) 85:104296. doi: 10.1016/j.ebiom.2022.104296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine (2020) 58:102925. doi: 10.1016/j.ebiom.2020.102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Blasco A, Coronado M-J, Hernández-Terciado F, Martín P, Royuela A, Ramil E, et al. Assessment of neutrophil extracellular traps in coronary thrombus of a case series of patients with COVID-19 and myocardial infarction. JAMA Cardiol (2021) 6(4):469–74. doi: 10.1001/jamacardio.2020.7308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr., et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA (2010) 107(36):15880–5. doi: 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood (2011) 118(13):3708–14. doi: 10.1182/blood-2011-01-332676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood (2011) 118(7):1952–61. doi: 10.1182/blood-2011-03-343061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood (2017) 129(10):1357–67. doi: 10.1182/blood-2016-09-741298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PloS One (2012) 7(2):e32366. doi: 10.1371/journal.pone.0032366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost (2014) 12(12):2074–88. doi: 10.1111/jth.12710 [DOI] [PubMed] [Google Scholar]
- 120. Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med (2010) 16(8):887–96. doi: 10.1038/nm.2184 [DOI] [PubMed] [Google Scholar]
- 121. Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest (2020) 130(11):6151–7. doi: 10.1172/JCI141374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, Forsyth PA, et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe (2013) 13(2):169–80. doi: 10.1016/j.chom.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 123. Ackermann M, Anders H-J, Bilyy R, Bowlin GL, Daniel C, De Lorenzo R, et al. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ (2021) 28(11):3125–39. doi: 10.1038/s41418-021-00805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Leung HHL, Perdomo J, Ahmadi Z, Zheng SS, Rashid FN, Enjeti A, et al. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat Commun (2022) 13(1):5206. doi: 10.1038/s41467-022-32946-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kashir J, Ambia AR, Shafqat A, Sajid MR, AlKattan K, Yaqinuddin A. Scientific premise for the involvement of neutrophil extracellular traps (NETs) in vaccine-induced thrombotic thrombocytopenia (VITT). J Leukoc Biol (2022) 111(3):725–34. doi: 10.1002/JLB.5COVR0621-320RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Greinacher A, Selleng K, Mayerle J, Palankar R, Wesche J, Reiche S, et al. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood (2021) 138(14):1269–77. doi: 10.1182/blood.2021012938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Afzali B, Noris M, Lambrecht BN, Kemper C. The state of complement in COVID-19. Nat Rev Immunol (2022) 22(2):77–84. doi: 10.1038/s41577-021-00665-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe (2012) 12(1):109–16. doi: 10.1016/j.chom.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 129. Hiroki CH, Toller-Kawahisa JE, Fumagalli MJ, Colon DF, Figueiredo LTM, Fonseca B, et al. Neutrophil extracellular traps effectively control acute chikungunya virus infection. Front Immunol (2019) 10:3108. doi: 10.3389/fimmu.2019.03108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Schauer C, Janko C, Munoz LE, Zhao Y, Kienhöfer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med (2014) 20(5):511–7. doi: 10.1038/nm.3547 [DOI] [PubMed] [Google Scholar]
- 131. Singh J, Boettcher M, Dölling M, Heuer A, Hohberger B, Leppkes M, et al. Moonlighting chromatin: when DNA escapes nuclear control. Cell Death Differ (2023) 30(4):861–75. doi: 10.1038/s41418-023-01124-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Scozzi D, Wang X, Liao F, Liu Z, Zhu J, Pugh K, et al. Neutrophil extracellular trap fragments stimulate innate immune responses that prevent lung transplant tolerance. Am J Transplantation (2019) 19(4):1011–23. doi: 10.1111/ajt.15163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ribon M, Seninet S, Mussard J, Sebbag M, Clavel C, Serre G, et al. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J Autoimmunity (2019) 98:122–31. doi: 10.1016/j.jaut.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 134. Guimarães-Costa AB, Rochael NC, Oliveira F, Echevarria-Lima J, Saraiva EM. Neutrophil extracellular traps reprogram IL-4/GM-CSF-induced monocyte differentiation to anti-inflammatory macrophages. Front Immunol (2017) 8:523. doi: 10.3389/fimmu.2017.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Eghbalzadeh K, Georgi L, Louis T, Zhao H, Keser U, Weber C, et al. Compromised anti-inflammatory action of neutrophil extracellular traps in PAD4-deficient mice contributes to aggravated acute inflammation after myocardial infarction. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.02313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Holly RK, James DC. Neutrophil extracellular traps in chronic lung disease: implications for pathogenesis and therapy. Eur Respir Review (2022) 31(163):210241. doi: 10.1183/16000617.0241-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wright TK, Gibson PG, Simpson JL, McDonald VM, Wood LG, Baines KJ. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology (2016) 21(3):467–75. doi: 10.1111/resp.12730 [DOI] [PubMed] [Google Scholar]
- 138. Ravi AK, Khurana S, Lemon J, Plumb J, Booth G, Healy L, et al. Increased levels of soluble interleukin-6 receptor and CCL3 in COPD sputum. Respir Res (2014) 15(1):103. doi: 10.1186/s12931-014-0103-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dicker AJ, Crichton ML, Pumphrey EG, Cassidy AJ, Suarez-Cuartin G, Sibila O, et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol (2018) 141(1):117–27. doi: 10.1016/j.jaci.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Dworski R, Simon H-U, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol (2011) 127(5):1260–6. doi: 10.1016/j.jaci.2010.12.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chen X, Li Y, Qin L, He R, Hu C. Neutrophil extracellular trapping network promotes the pathogenesis of neutrophil-associated asthma through macrophages. Immunol Invest (2021) 50(5):544–61. doi: 10.1080/08820139.2020.1778720 [DOI] [PubMed] [Google Scholar]
- 142. Twaddell SH, Baines KJ, Grainge C, Gibson PG. The emerging role of neutrophil extracellular traps in respiratory disease. Chest (2019) 156(4):774–82. doi: 10.1016/j.chest.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 143. Khan MA, Ali ZS, Sweezey N, Grasemann H, Palaniyar N. Progression of cystic fibrosis lung disease from childhood to adulthood: neutrophils, neutrophil extracellular trap (NET) formation, and NET degradation. Genes (Basel) (2019) 10(3):183. doi: 10.3390/genes10030183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Dwyer M, Shan Q, D'Ortona S, Maurer R, Mitchell R, Olesen H, et al. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J Innate Immun (2014) 6(6):765–79. doi: 10.1159/000363242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Papayannopoulos V, Staab D, Zychlinsky A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PloS One (2011) 6(12):e28526. doi: 10.1371/journal.pone.0028526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front Immunol (2016) 7:311. doi: 10.3389/fimmu.2016.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Marcos V, Zhou-Suckow Z, Önder Yildirim A, Bohla A, Hector A, Vitkov L, et al. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflammation (2015) 2015:408935. doi: 10.1155/2015/408935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Guerra M, Halls VS, Schatterny J, Hagner M, Mall MA, Schultz C. Protease FRET reporters targeting neutrophil extracellular traps. J Am Chem Soc (2020) 142(48):20299–305. doi: 10.1021/jacs.0c08130 [DOI] [PubMed] [Google Scholar]
- 149. Gehrig S, Duerr J, Weitnauer M, Wagner CJ, Graeber SY, Schatterny J, et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis–like lung disease. Am J Respir Crit Care Med (2014) 189(9):1082–92. doi: 10.1164/rccm.201311-1932OC [DOI] [PubMed] [Google Scholar]
- 150. Rada B, Jendrysik MA, Pang L, Hayes CP, Yoo DG, Park JJ, et al. Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase. PloS One (2013) 8(1):e54205. doi: 10.1371/journal.pone.0054205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yoo D-g, Floyd M, Winn M, Moskowitz SM, Rada B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase–DNA and neutrophil elastase–DNA complexes. Immunol Lett (2014) 160(2):186–94. doi: 10.1016/j.imlet.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 152. Podolska MJ, Mahajan A, Hahn J, Knopf J, Maueröder C, Petru L, et al. Treatment with DNases rescues hidden neutrophil elastase from aggregated NETs. J Leukocyte Biol (2019) 106(6):1359–66. doi: 10.1002/JLB.3AB0918-370R [DOI] [PubMed] [Google Scholar]
- 153. Cole P. Inflammation: a two-edged sword–the model of bronchiectasis. Eur J Respir Dis Supplement (1986) 147:6–15. [PubMed] [Google Scholar]
- 154. Keir HR, Shoemark A, Dicker AJ, Perea L, Pollock J, Giam YH, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med (2021) 9(8):873–84. doi: 10.1016/S2213-2600(20)30504-X [DOI] [PubMed] [Google Scholar]
- 155. Sabbione F, Keitelman IA, Iula L, Ferrero M, Giordano MN, Baldi P, et al. Neutrophil extracellular traps stimulate proinflammatory responses in human airway epithelial cells. J Innate Immun (2017) 9(4):387–402. doi: 10.1159/000460293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hudock KM, Collins MS, Imbrogno M, Snowball J, Kramer EL, Brewington JJ, et al. Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol (2020) 319(1):L137–l47. doi: 10.1152/ajplung.00144.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zou Y, Chen X, He B, Xiao J, Yu Q, Xie B, et al. Neutrophil extracellular traps induced by cigarette smoke contribute to airway inflammation in mice. Exp Cell Res (2020) 389(1):111888. doi: 10.1016/j.yexcr.2020.111888 [DOI] [PubMed] [Google Scholar]
- 158. Zhang H, Qiu S-L, Tang Q-Y, Zhou X, Zhang J-Q, He Z-Y, et al. Erythromycin suppresses neutrophil extracellular traps in smoking-related chronic pulmonary inflammation. Cell Death Dis (2019) 10(9):678. doi: 10.1038/s41419-019-1909-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest (2012) 122(7):2661–71. doi: 10.1172/JCI61303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med (2013) 187(2):160–9. doi: 10.1164/rccm.201206-1037OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Li H, Pan P, Su X, Liu S, Zhang L, Wu D, et al. Neutrophil extracellular traps are pathogenic in ventilator-induced lung injury and partially dependent on TLR4. BioMed Res Int (2017) 2017:827504. doi: 10.1155/2017/8272504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Sayah DM, Mallavia B, Liu F, Ortiz-Muñoz G, Caudrillier A, DerHovanessian A, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med (2014) 191(4):455–63. doi: 10.1164/rccm.201406-1086OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Liu S, Su X, Pan P, Zhang L, Hu Y, Tan H, et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep (2016) 6(1):1–8. doi: 10.1038/srep37252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew A-A, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol (2011) 179(1):199–210. doi: 10.1016/j.ajpath.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yaqinuddin A, Kashir J. Novel therapeutic targets for SARS-CoV-2-induced acute lung injury: Targeting a potential IL-1β/neutrophil extracellular traps feedback loop. Med Hypotheses (2020) 143:109906. doi: 10.1016/j.mehy.2020.109906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Yaqinuddin A, Kvietys P, Kashir J. COVID-19: Role of neutrophil extracellular traps in acute lung injury. Respir Invest (2020) 58(5):419. doi: 10.1016/j.resinv.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Scozzi D, Liao F, Krupnick AS, Kreisel D, Gelman AE. The role of neutrophil extracellular traps in acute lung injury. Front Immunol (2022) 13:953195. doi: 10.3389/fimmu.2022.953195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Tadie J-M, Bae H-B, Jiang S, Park DW, Bell CP, Yang H, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiology-Lung Cell Mol Physiol (2013) 304(5):L342–L9. doi: 10.1152/ajplung.00151.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Zhang H, Zhou Y, Qu M, Yu Y, Chen Z, Zhu S, et al. Tissue factor-enriched neutrophil extracellular traps promote immunothrombosis and disease progression in sepsis-induced lung injury. Front Cell Infect Microbiol (2021) 11:677902. doi: 10.3389/fcimb.2021.677902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Yan S, Li M, Liu B, Ma Z, Yang Q. Neutrophil extracellular traps and pulmonary fibrosis: an update. J Inflamm (2023) 20(1):2. doi: 10.1186/s12950-023-00329-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol (2014) 233(3):294–307. doi: 10.1002/path.4359 [DOI] [PubMed] [Google Scholar]
- 172. Suzuki M, Ikari J, Anazawa R, Tanaka N, Katsumata Y, Shimada A, et al. PAD4 deficiency improves bleomycin-induced neutrophil extracellular traps and fibrosis in mouse lung. Am J Respir Cell Mol Biol (2020) 63(6):806–18. doi: 10.1165/rcmb.2019-0433OC [DOI] [PubMed] [Google Scholar]
- 173. Zhang S, Jia X, Zhang Q, Zhang L, Yang J, Hu C, et al. Neutrophil extracellular traps activate lung fibroblast to induce polymyositis-related interstitial lung diseases via TLR9-miR-7-Smad2 pathway. J Cell Mol Med (2020) 24(2):1658–69. doi: 10.1111/jcmm.14858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Gregory AD, Kliment CR, Metz HE, Kim K-H, Kargl J, Agostini BA, et al. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J Leukocyte Biol (2015) 98(2):143–52. doi: 10.1189/jlb.3HI1014-493R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Wang YY, Jiang H, Pan J, Huang XR, Wang YC, Huang HF, et al. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J Am Soc Nephrol (2017) 28(7):2053–67. doi: 10.1681/ASN.2016050573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jin Z, Sun J, Song Z, Chen K, Nicolas YSM, Kc R, et al. Neutrophil extracellular traps promote scar formation in post-epidural fibrosis. NPJ Regenerative Med (2020) 5(1):19. doi: 10.1038/s41536-020-00103-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bozzini S, Pandolfi L, Vertui V, Frangipane V, Violatto M, De Luigi A, et al. Induction of epithelial-mesenchymal transition (EMT) by neutrophil extracellular traps (NETs) as possible molecular mechanism in CLAD. J Heart Lung Transplantation (2021) 40(4, Supplement):S151. doi: 10.1016/j.healun.2021.01.460 [DOI] [Google Scholar]
- 178. Pieterse E, Rother N, Garsen M, Hofstra JM, Satchell SC, Hoffmann M, et al. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arteriosclerosis Thrombosis Vasc Biol (2017) 37(7):1371–9. doi: 10.1161/ATVBAHA.117.309002 [DOI] [PubMed] [Google Scholar]
- 179. Wang Y, Li Y, Chen Z, Yuan Y, Su Q, Ye K, et al. GSDMD-dependent neutrophil extracellular traps promote macrophage-to-myofibroblast transition and renal fibrosis in obstructive nephropathy. Cell Death Dis (2022) 13(8):693. doi: 10.1038/s41419-022-05138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Sharma S, Hofbauer TM, Ondracek AS, Chausheva S, Alimohammadi A, Artner T, et al. Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood (2021) 137(8):1104–16. doi: 10.1182/blood.2020005861 [DOI] [PubMed] [Google Scholar]
- 181. Sawadogo SA, Dighero-Kemp B, Ouédraogo DD, Hensley L, Sakandé J. How NETosis could drive "Post-COVID-19 syndrome" among survivors. Immunol Lett (2020) 228:35–7. doi: 10.1016/j.imlet.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Karmakar U, Vermeren S. Crosstalk between B cells and neutrophils in rheumatoid arthritis. Immunology (2021) 164(4):689–700. doi: 10.1111/imm.13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yaqinuddin A, Kashir J. The central role of neutrophil extracellular traps in SARS-CoV-2-induced thrombogenesis and vasculitis. Afr J Respir Med (2020) 15(2). [Google Scholar]
- 184. Krinsky N, Sizikov S, Nissim S, Dror A, Sas A, Prinz H, et al. NETosis induction reflects COVID-19 severity and long COVID: insights from a 2-center patient cohort study in Israel. J Thromb Haemostasis (2023) 21(9):2569–84. doi: 10.1016/j.jtha.2023.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Woodruff MC, Bonham KS, Anam FA, Walker TA, Faliti CE, Ishii Y, et al. Chronic inflammation, neutrophil activity, and autoreactivity splits long COVID. Nat Commun (2023) 14:14201. doi: 10.1038/s41467-023-40012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Pisareva E, Badiou S, Mihalovičová L, Mirandola A, Pastor B, Kudriavtsev A, et al. Persistence of neutrophil extracellular traps and anticardiolipin auto-antibodies in post-acute phase COVID-19 patients. J Med Virol (2023) 95(1):e28209. doi: 10.1002/jmv.28209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Pascolini S, Vannini A, Deleonardi G, Ciordinik M, Sensoli A, Carletti I, et al. COVID-19 and immunological dysregulation: can autoantibodies be useful? Clin Trans Science (2021) 14(2):502–8. doi: 10.1111/cts.12908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Kadkhoda K, Laurita K. Antineutrophil cytoplasmic antibodies and their association with clinical outcomes in hospitalized COVID-19 patients. Cell Death Discov (2021) 7(1):277. doi: 10.1038/s41420-021-00671-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Chang SE, Feng A, Meng W, Apostolidis SA, Mack E, Artandi M, et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat Commun (2021) 12(1):5417. doi: 10.1038/s41467-021-25509-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science (2020) 370(6515):eabd4585. doi: 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Vahabi M, Mirsharif ES, Ghazanfari T. Is COVID-19 severity unrelated to antinuclear antibodies? Transpl Immunol (2023) 78:101791. doi: 10.1016/j.trim.2023.101791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zuo Y, Yalavarthi S, Navaz SA, Hoy CK, Harbaugh A, Gockman K, et al. Autoantibodies stabilize neutrophil extracellular traps in COVID-19. JCI Insight (2021) 6(15):e150111. doi: 10.1172/jci.insight.150111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA (2010) 107(21):9813–8. doi: 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol (2020) 21(12):1506–16. doi: 10.1038/s41590-020-00814-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Laidlaw BJ, Ellebedy AH. The germinal centre B cell response to SARS-CoV-2. Nat Rev Immunol (2022) 22(1):7–18. doi: 10.1038/s41577-021-00657-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Wang EY, Mao T, Klein J, Dai Y, Huck JD, Jaycox JR, et al. Diverse functional autoantibodies in patients with COVID-19. Nature (2021) 595(7866):283–8. doi: 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- 197. Taghadosi M, Safarzadeh E, Asgarzadeh A, Roghani SA, Shamsi A, Jalili C, et al. Partners in crime: Autoantibodies complicit in COVID-19 pathogenesis. Rev Med Virol (2023) 33(2):e2412. doi: 10.1002/rmv.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kiho S, Rameen J, Abhiroop C, Manan M, Carmen V, Kate M, et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J (2023) 61(1):2200970. doi: 10.1183/13993003.00970-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zuo Y, Estes SK, Ali RA, Gandhi AA, Yalavarthi S, Shi H, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Trans Med (2020) 12(570):eabd3876. doi: 10.1126/scitranslmed.abd3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sørensen AIV, Spiliopoulos L, Bager P, Nielsen NM, Hansen JV, Koch A, et al. A nationwide questionnaire study of post-acute symptoms and health problems after SARS-CoV-2 infection in Denmark. Nat Commun (2022) 13(1):4213. doi: 10.1038/s41467-022-31897-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature (2021) 594(7862):259–64. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 202. Alkodaymi MS, Omrani OA, Fawzy NA, Shaar BA, Almamlouk R, Riaz M, et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: a systematic review and meta-analysis. Clin Microbiol Infect (2022) 28(5):657–66. doi: 10.1016/j.cmi.2022.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Achkar M, Jamal O, Chaaban T. Post-COVID lung disease(s). Ann Thorac Med (2022) 17(3):137–44. doi: 10.4103/atm.atm_103_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Caruso D, Guido G, Zerunian M, Polidori T, Lucertini E, Pucciarelli F, et al. Post-acute sequelae of COVID-19 pneumonia: six-month chest CT follow-up. Radiology (2021) 301(2):E396–405. doi: 10.1148/radiol.2021210834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Hama Amin BJ, Kakamad FH, Ahmed GS, Ahmed SF, Abdulla BA, Mohammed SH, et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond) (2022) 77:103590. doi: 10.1016/j.amsu.2022.103590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. George PM, Reed A, Desai SR, Devaraj A, Faiez TS, Laverty S, et al. A persistent neutrophil-associated immune signature characterizes post–COVID-19 pulmonary sequelae. Sci Trans Med (2022) 14(671):eabo5795. doi: 10.1126/scitranslmed.abo5795 [DOI] [PubMed] [Google Scholar]
- 207. Pandolfi L, Bozzini S, Frangipane V, Percivalle E, De Luigi A, Violatto MB, et al. Neutrophil extracellular traps induce the epithelial-mesenchymal transition: implications in post-COVID-19 fibrosis. Front Immunol (2021) 12. doi: 10.3389/fimmu.2021.663303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Chrysanthopoulou A, Antoniadou C, Natsi A-M, Gavriilidis E, Papadopoulos V, Xingi E, et al. Down-regulation of KLF2 in lung fibroblasts is linked with COVID-19 immunofibrosis and restored by combined inhibition of NETs, JAK-1/2 and IL-6 signaling. Clin Immunol (2023) 247:109240. doi: 10.1016/j.clim.2023.109240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Shi J, Zhou LR, Wang XS, Du JF, Jiang MM, Song Z, et al. KLF2 attenuates bleomycin-induced pulmonary fibrosis and inflammation with regulation of AP-1. Biochem Biophys Res Commun (2018) 495(1):20–6. doi: 10.1016/j.bbrc.2017.10.114 [DOI] [PubMed] [Google Scholar]
- 210. Li H, Wang Y, Liu J, Chen X, Duan Y, Wang X, et al. Endothelial Klf2-Foxp1-TGFβ signal mediates the inhibitory effects of simvastatin on maladaptive cardiac remodeling. Theranostics (2021) 11(4):1609–25. doi: 10.7150/thno.48153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med (2022) 28(3):583–90. doi: 10.1038/s41591-022-01689-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Knight R, Walker V, Ip S, Cooper JA, Bolton T, Keene S, et al. Association of COVID-19 with major arterial and venous thrombotic diseases: A population-wide cohort study of 48 million adults in england and wales. Circulation (2022) 146(12):892–906. doi: 10.1161/CIRCULATIONAHA.122.060785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Sollini M, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, Gelardi F, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [(18)F]FDG-PET/CT study. Eur J Nucl Med Mol Imag (2021) 48(5):1460–6. doi: 10.1007/s00259-020-05084-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol (2021) 20(1):172. doi: 10.1186/s12933-021-01359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. McFadyen JD, Stevens H, Peter K. The emerging threat of (Micro)Thrombosis in COVID-19 and its therapeutic implications. Circ Res (2020) 127(4):571–87. doi: 10.1161/CIRCRESAHA.120.317447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC). Cardiovasc Diabetol (2022) 21(1):148. doi: 10.1186/s12933-022-01579-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol (2020) 20(7):389–91. doi: 10.1038/s41577-020-0343-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Siddiqi HK, Libby P, Ridker PM. COVID-19 - A vascular disease. Trends Cardiovasc Med (2021) 31(1):1–5. doi: 10.1016/j.tcm.2020.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Turner S, Naidoo CA, Usher TJ, Kruger A, Venter C, Laubscher GJ, et al. Increased levels of inflammatory and endothelial biomarkers in blood of long COVID patients point to thrombotic endothelialitis. Semin Thromb Hemost (2023). doi: 10.1055/s-0043-1769014 [DOI] [PubMed] [Google Scholar]
- 220. Hocini H, Wiedemann A, Blengio F, Lefebvre C, Cervantes-Gonzalez M, Foucat E, et al. Neutrophil activation and immune thrombosis profiles persist in convalescent COVID-19. J Clin Immunol (2023) 43(5):882–93. doi: 10.1007/s10875-023-01459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Grover SP, Mackman N. Neutrophils, NETs, and immunothrombosis. Blood (2018) 132(13):1360–1. doi: 10.1182/blood-2018-08-868067 [DOI] [PubMed] [Google Scholar]
- 222. Zhu Y, Chen X, Liu X. NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and beyond. Front Immunol (2022) 13:838011. doi: 10.3389/fimmu.2022.838011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett (2010) 584(14):3193–7. doi: 10.1016/j.febslet.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 224. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science (2015) 349(6245):316–20. doi: 10.1126/science.aaa8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Qi H, Yang S, Zhang L. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front Immunol (2017) 8:928. doi: 10.3389/fimmu.2017.00928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Döring Y, Libby P, Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res (2020) 126(9):1228–41. doi: 10.1161/CIRCRESAHA.120.315931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res (2017) 120(4):736–43. doi: 10.1161/CIRCRESAHA.116.309692 [DOI] [PubMed] [Google Scholar]
- 228. Shafqat A, Abdul Rab S, Ammar O, Al Salameh S, Alkhudairi A, Kashir J, et al. Emerging role of neutrophil extracellular traps in the complications of diabetes mellitus. Front Med (2022) 9. doi: 10.3389/fmed.2022.995993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Matsumoto K, Yasuoka H, Yoshimoto K, Suzuki K, Takeuchi T. Platelet CXCL4 mediates neutrophil extracellular traps formation in ANCA-associated vasculitis. Sci Rep (2021) 11(1):222. doi: 10.1038/s41598-020-80685-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res (2014) 114(6):947–56. doi: 10.1161/CIRCRESAHA.114.303312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Liu Y, Carmona-Rivera C, Moore E, Seto NL, Knight JS, Pryor M, et al. Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front Immunol (2018) 9:1680. doi: 10.3389/fimmu.2018.01680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Ge L, Zhou X, Ji WJ, Lu RY, Zhang Y, Zhang YD, et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol (2015) 308(5):H500–9. doi: 10.1152/ajpheart.00381.2014 [DOI] [PubMed] [Google Scholar]
- 233. O'Sullivan KM, Holdsworth SR. Neutrophil extracellular traps: A potential therapeutic target in MPO-ANCA associated vasculitis? Front Immunol (2021) 12:635188. doi: 10.3389/fimmu.2021.635188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Blasco A, Coronado MJ, Hernández-Terciado F, Martín P, Royuela A, Ramil E, et al. Assessment of neutrophil extracellular traps in coronary thrombus of a case series of patients with COVID-19 and myocardial infarction. JAMA Cardiol (2020) 6(4):1–6. doi: 10.1001/jamacardio.2020.7308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Tobler DL, Pruzansky AJ, Naderi S, Ambrosy AP, Slade JJ. Long-term cardiovascular effects of COVID-19: emerging data relevant to the cardiovascular clinician. Curr Atheroscler Rep (2022) 24(7):563–70. doi: 10.1007/s11883-022-01032-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Satterfield BA, Bhatt DL, Gersh BJ. Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol (2022) 19(5):332–41. doi: 10.1038/s41569-021-00631-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Abbasi J. The COVID heart-one year after SARS-coV-2 infection, patients have an array of increased cardiovascular risks. Jama (2022) 327(12):1113–4. doi: 10.1001/jama.2022.2411 [DOI] [PubMed] [Google Scholar]
- 238. Davoudi F, Miyashita S, Yoo TK, Lee PT, Foster GP. An insight into pathophysiology, epidemiology, and management of cardiovascular complications of SARS-coV-2 infection, post-acute COVID syndrome, and COVID vaccine. Crit Pathw Cardiol (2022) 21(3):123–9. doi: 10.1097/HPC.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 239. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol (2020) 5(11):1265–73. doi: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Krishnan A, Ellenberger KA, Phetsouphanh C, Kelleher AP, Matthews GV, Darley DR, et al. Myocardial fibrosis occurs in non-hospitalised patients with chronic symptoms after COVID-19. Int J Cardiol Heart Vasc (2022) 39:100964. doi: 10.1016/j.ijcha.2022.100964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Thakur M, Evans B, Schindewolf M, Baumgartner I, Döring Y. Neutrophil extracellular traps affecting cardiovascular health in infectious and inflammatory diseases. Cells (2021) 10(7):1689. doi: 10.3390/cells10071689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Hanson PJ, Liu-Fei F, Ng C, Minato TA, Lai C, Hossain AR, et al. Characterization of COVID-19-associated cardiac injury: evidence for a multifactorial disease in an autopsy cohort. Lab Invest (2022) 102(8):814–25. doi: 10.1038/s41374-022-00783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Veras FP, Gomes GF, Silva BMS, Caetité DB, Almeida C, Silva CMS, et al. Targeting neutrophils extracellular traps (NETs) reduces multiple organ injury in a COVID-19 mouse model. Respir Res (2023) 24(1):66. doi: 10.1186/s12931-023-02336-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Weckbach LT, Grabmaier U, Uhl A, Gess S, Boehm F, Zehrer A, et al. Midkine drives cardiac inflammation by promoting neutrophil trafficking and NETosis in myocarditis. J Exp Med (2019) 216(2):350–68. doi: 10.1084/jem.20181102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. He L, Liu R, Yue H, Zhu G, Fu L, Chen H, et al. NETs promote pathogenic cardiac fibrosis and participate in ventricular aneurysm formation after ischemia injury through the facilitation of perivascular fibrosis. Biochem Biophys Res Commun (2021) 583:154–61. doi: 10.1016/j.bbrc.2021.10.068 [DOI] [PubMed] [Google Scholar]
- 246. Navis A. A review of neurological symptoms in long COVID and clinical management. Semin Neurol (2023) 43(2):286–96. doi: 10.1055/s-0043-1767781 [DOI] [PubMed] [Google Scholar]
- 247. Stefanou MI, Palaiodimou L, Bakola E, Smyrnis N, Papadopoulou M, Paraskevas GP, et al. Neurological manifestations of long-COVID syndrome: a narrative review. Ther Adv Chronic Dis (2022) 13:20406223221076890. doi: 10.1177/20406223221076890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Sobrino-Relaño S, Balboa-Bandeira Y, Peña J, Ibarretxe-Bilbao N, Zubiaurre-Elorza L, Ojeda N. Neuropsychological deficits in patients with persistent COVID-19 symptoms: a systematic review and meta-analysis. Sci Rep (2023) 13(1):10309. doi: 10.1038/s41598-023-37420-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med (2022) 28(11):2406–15. doi: 10.1038/s41591-022-02001-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature (2022) 604(7907):697–707. doi: 10.1038/s41586-022-04569-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Monje M, Iwasaki A. The neurobiology of long COVID. Neuron (2022) 110(21):3484–96. doi: 10.1016/j.neuron.2022.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Venkataramani V, Winkler F. Cognitive deficits in long covid-19. New Engl J Med (2022) 387(19):1813–5. doi: 10.1056/NEJMcibr2210069 [DOI] [PubMed] [Google Scholar]
- 253. Braga J, Lepra M, Kish SJ, Rusjan PM, Nasser Z, Verhoeff N, et al. Neuroinflammation after COVID-19 with persistent depressive and cognitive symptoms. JAMA Psychiatry (2023) 80(8):787–95. doi: 10.1001/jamapsychiatry.2023.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Campbell M, Greene C, Connolly R, Brennan D, Laffan A, O'Keeffe E, et al. Blood-brain barrier disruption in Long COVID-associated cognitive impairment. (2022). doi: 10.21203/rs.3.rs-2069710/v1 [DOI] [Google Scholar]
- 255. Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, et al. Microvascular injury in the brains of patients with covid-19. N Engl J Med (2021) 384(5):481–3. doi: 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lee MH, Perl DP, Steiner J, Pasternack N, Li W, Maric D, et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain (2022) 145(7):2555–68. doi: 10.1093/brain/awac151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Taquet M, Skorniewska Z, Hampshire A, Chalmers JD, Ho L-P, Horsley A, et al. Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID-19 hospitalization. Nat Med (2023). doi: 10.1038/s41591-023-02525-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood (2011) 117(18):4953–63. doi: 10.1182/blood-2010-11-316885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Petersen MA, Ryu JK, Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci (2018) 19(5):283–301. doi: 10.1038/nrn.2018.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Santos-Lima B, Pietronigro EC, Terrabuio E, Zenaro E, Constantin G. The role of neutrophils in the dysfunction of central nervous system barriers. Front Aging Neurosci (2022) 14. doi: 10.3389/fnagi.2022.965169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Shafqat A, Noor Eddin A, Adi G, Al-Rimawi M, Abdul Rab S, Abu-Shaar M, et al. Neutrophil extracellular traps in central nervous system pathologies: A mini review. Front Med (2023) 10. doi: 10.3389/fmed.2023.1083242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Rosell A, Cuadrado E, Ortega-Aznar A, Hernández-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke (2008) 39(4):1121–6. doi: 10.1161/STROKEAHA.107.500868 [DOI] [PubMed] [Google Scholar]
- 263. Hernández-Parra H, Reyes-Hernández OD, Figueroa-González G, González-Del Carmen M, González-Torres M, Peña-Corona SI, et al. Alteration of the blood-brain barrier by COVID-19 and its implication in the permeation of drugs into the brain. Front Cell Neurosci (2023) 17:1125109. doi: 10.3389/fncel.2023.1125109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Zenaro E, Pietronigro E, Bianca VD, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer's disease–like pathology and cognitive decline via LFA-1 integrin. Nat Med (2015) 21(8):880–6. doi: 10.1038/nm.3913 [DOI] [PubMed] [Google Scholar]
- 265. Silva LM, Doyle AD, Greenwell-Wild T, Dutzan N, Tran CL, Abusleme L, et al. Fibrin is a critical regulator of neutrophil effector function at the oral mucosal barrier. Science (2021) 374(6575):eabl5450. doi: 10.1126/science.abl5450 [DOI] [PubMed] [Google Scholar]
- 266. Yang J, Wu Z, Long Q, Huang J, Hong T, Liu W, et al. Insights into immunothrombosis: the interplay among neutrophil extracellular trap, von willebrand factor, and ADAMTS13. Front Immunol (2020) 11:610696. doi: 10.3389/fimmu.2020.610696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Kim SJ, Jenne CN. Role of platelets in neutrophil extracellular trap (NET) production and tissue injury. Semin Immunol (2016) 28(6):546–54. doi: 10.1016/j.smim.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 268. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res (1961) 25(3):585–621. doi: 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- 269. Shafqat S, Arana Chicas E, Shafqat A, Hashmi SK. The Achilles’ heel of cancer survivors: fundamentals of accelerated cellular senescence. J Clin Invest (2022) 132(13):e158452. doi: 10.1172/JCI158452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell (2023) 186(2):243–78. doi: 10.1016/j.cell.2022.11.001 [DOI] [PubMed] [Google Scholar]
- 271. Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. Cellular senescence: defining a path forward. Cell (2019) 179(4):813–27. doi: 10.1016/j.cell.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 272. Evangelou K, Vasileiou PVS, Papaspyropoulos A, Hazapis O, Petty R, Demaria M, et al. Cellular senescence and cardiovascular diseases: moving to the "heart" of the problem. Physiol Rev (2023) 103(1):609–47. doi: 10.1152/physrev.00007.2022 [DOI] [PubMed] [Google Scholar]
- 273. Guerrero A, De Strooper B, Arancibia-Cárcamo IL. Cellular senescence at the crossroads of inflammation and Alzheimer's disease. Trends Neurosci (2021) 44(9):714–27. doi: 10.1016/j.tins.2021.06.007 [DOI] [PubMed] [Google Scholar]
- 274. Arabi T, Shafqat A, Sabbah BN, Fawzy NA, Shah H, Abdulkader H, et al. Obesity-related kidney disease: Beyond hypertension and insulin-resistance. Front Endocrinol (2023) 13:1095211. doi: 10.3389/fendo.2022.1095211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Aversa Z, Atkinson EJ, Carmona EM, White TA, Heeren AA, Jachim SK, et al. Biomarkers of cellular senescence in idiopathic pulmonary fibrosis. Respir Res (2023) 24(1):101. doi: 10.1186/s12931-023-02403-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Di Micco R, Krizhanovsky V, Baker D, d’Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol (2021) 22(2):75–95. doi: 10.1038/s41580-020-00314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest (2018) 128(4):1238–46. doi: 10.1172/JCI95148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Schmitt CA, Tchkonia T, Niedernhofer LJ, Robbins PD, Kirkland JL, Lee S. COVID-19 and cellular senescence. Nat Rev Immunol (2023) 23(4):251–63. doi: 10.1038/s41577-022-00785-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Evangelou K, Veroutis D, Paschalaki K, Foukas PG, Lagopati N, Dimitriou M, et al. Pulmonary infection by SARS-CoV-2 induces senescence accompanied by an inflammatory phenotype in severe COVID-19: possible implications for viral mutagenesis. Eur Respir J (2022) 60(2):2102951. doi: 10.1183/13993003.02951-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Lee S, Yu Y, Trimpert J, Benthani F, Mairhofer M, Richter-Pechanska P, et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature (2021) 599(7884):283–9. doi: 10.1038/s41586-021-03995-1 [DOI] [PubMed] [Google Scholar]
- 281. D’Agnillo F, Walters K-A, Xiao Y, Sheng Z-M, Scherler K, Park J, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Trans Med (2021) 13(620):eabj7790. doi: 10.1126/scitranslmed.abj7790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Tsuji S, Minami S, Hashimoto R, Konishi Y, Suzuki T, Kondo T, et al. SARS-CoV-2 infection triggers paracrine senescence and leads to a sustained senescence-associated inflammatory response. Nat Aging. (2022) 2(2):115–24. doi: 10.1038/s43587-022-00170-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature (2011) 479(7374):547–51. doi: 10.1038/nature10599 [DOI] [PubMed] [Google Scholar]
- 284. Chibaya L, Snyder J, Ruscetti M. Senescence and the tumor-immune landscape: implications for cancer immunotherapy. Semin Cancer Biol (2022) 6(Pt 3):827–45. doi: 10.1016/j.semcancer.2022.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Yasuda T, Baba H, Ishimoto T. Cellular senescence in the tumor microenvironment and context-specific cancer treatment strategies. FEBS J (2023) 290(5):1290–302. doi: 10.1111/febs.16231 [DOI] [PubMed] [Google Scholar]
- 286. Hoenicke L, Zender L. Immune surveillance of senescent cells—biological significance in cancer-and non-cancer pathologies. Carcinogenesis (2012) 33(6):1123–6. doi: 10.1093/carcin/bgs124 [DOI] [PubMed] [Google Scholar]
- 287. Julio A, Alberto AA, Atefeh Taherian F, Eduardo AA, Alexander T, Marius S, et al. Senolytic therapy alleviates physiological human brain aging and COVID-19 neuropathology. bioRxiv (2023) 2023:01.17.524329. doi: 10.1101/2023.01.17.524329 [DOI] [Google Scholar]
- 288. Wissler Gerdes EO, Vanichkachorn G, Verdoorn BP, Hanson GJ, Joshi AY, Murad MH, et al. Role of senescence in the chronic health consequences of COVID-19. Transl Res (2022) 241:96–108. doi: 10.1016/j.trsl.2021.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Sfera A, Osorio C, Zapata Martín Del Campo CM, Pereida S, Maurer S, Maldonado JC, et al. Endothelial senescence and chronic fatigue syndrome, a COVID-19 based hypothesis. Front Cell Neurosci (2021) 15:673217. doi: 10.3389/fncel.2021.673217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Mohandas S, Jagannathan P, Henrich TJ, Sherif ZA, Bime C, Quinlan E, et al. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife (2023) 12:e86014. doi: 10.7554/eLife.86014 [DOI] [PMC free article] [PubMed] [Google Scholar]