Abstract
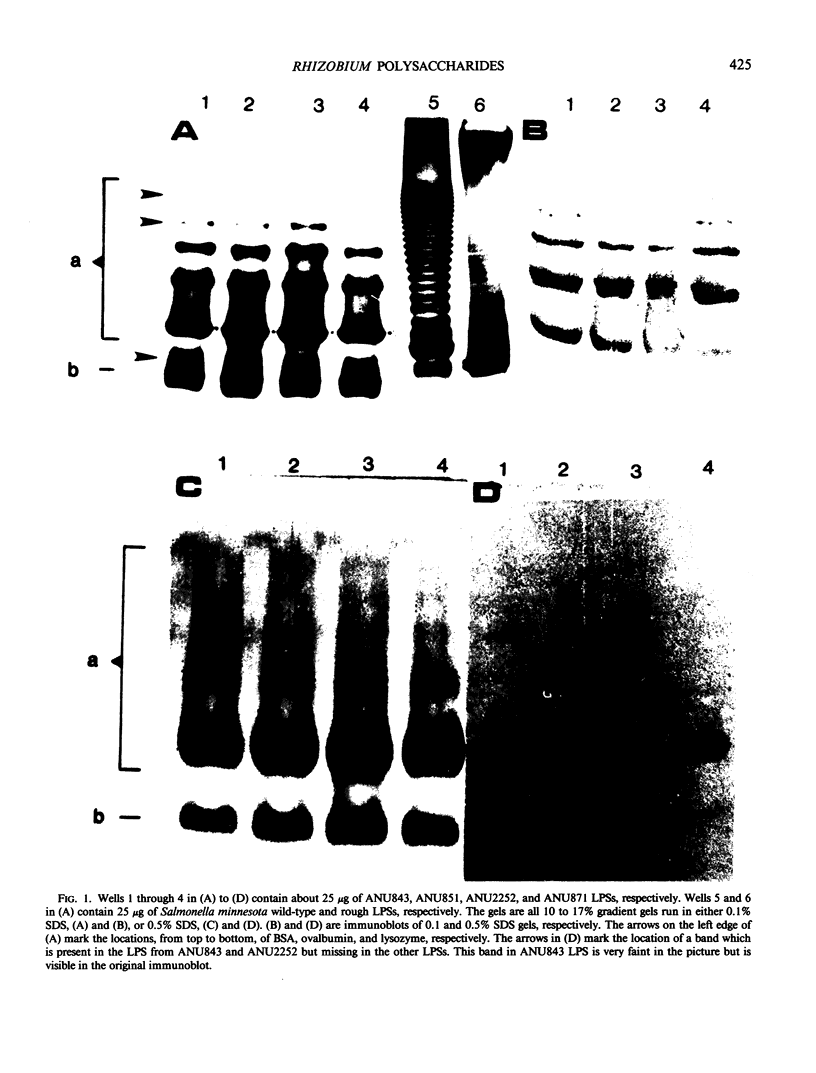
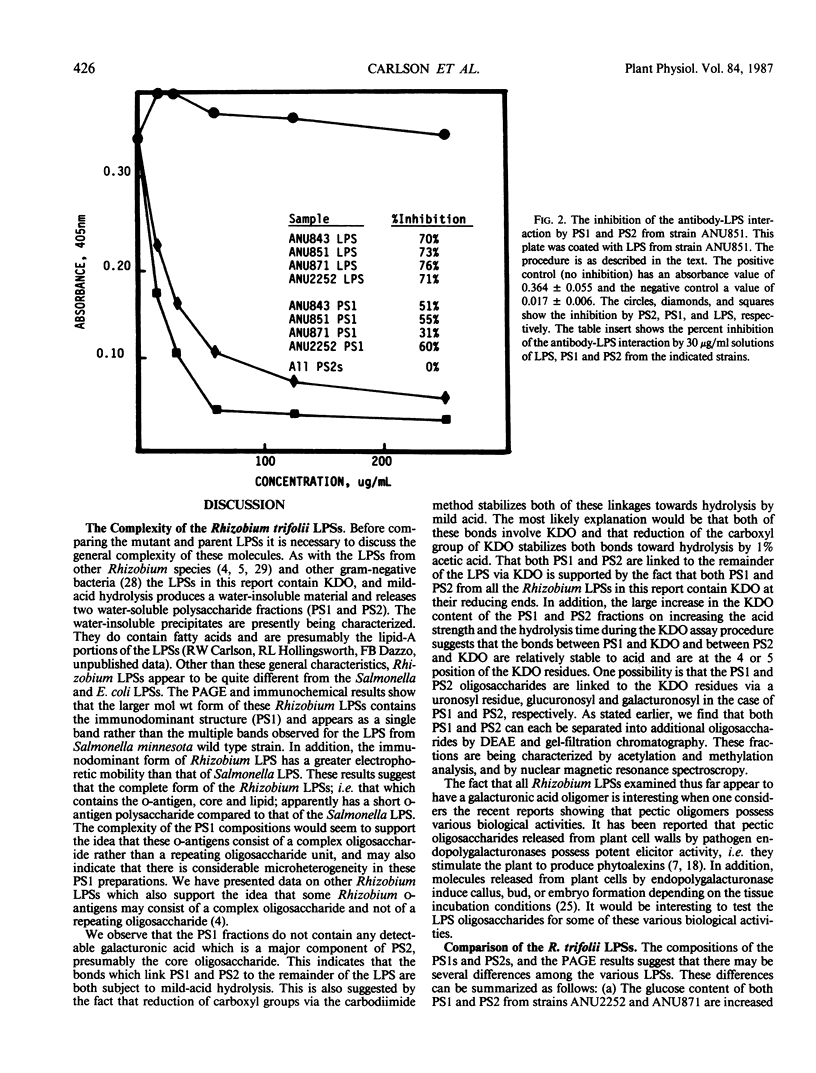
The lipopolysaccharides (LPSs) from Rhizobium trifolii ANU843 and several transposon (Tn5) symbiotic mutants derived from ANU843 were isolated and partially characterized. The mutant strains are unable to induce normal root hair curling (Hac- phenotype) or nodulation (Nod-phenotype) in clover plants. The LPSs from the parent and mutants are very similar in composition. Analysis by PAGE shows that the LPSs consist of higher and lower molecular weight forms. The higher molecular weight form of the LPSs exists in several aggregation states when PAGE is done in 0.1% SDS but collapses into a single band when PAGE is done in 0.5% SDS. Mild acid hydrolysis of all the LPSs releases two polysaccharides, PS1 and PS2. Immunoblots of the PAGE gels and enzyme linked immunosorbant assay inhibition assays show that the PS1 fractions contain the immunodominant sites of the LPSs and that these sites are present in the higher molecular weight form of the LPSs. All the PS1 fractions contain methylated sugars, 2-amino-2,6-dideoxyhexose, heptose, glucuronic acid, and 2-keto-3-deoxyoctonic acid (KDO). All the PS2 fractions contain galacturonic acid, mannose, galactose, and KDO. The PS2 fractions have a molecular weight of about 700. The KDO is present at the reducing end of both the PS1 and the PS2 fractions. The PS1 and PS2 fractions from the mutants contain more glucose than these fractions from the parent. The LPS from a deletion mutant contains less acyl groups than the other LPSs. Immunoblots of the LPSs show that the parent and nod A mutant LPSs contain an additional antigenic band which is not observed in the other LPSs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Carlson R. W. Heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984 Jun;158(3):1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Shaw D. H., Ishiguro E. E., Trust T. J. Structural and immunochemical homogeneity of Aeromonas salmonicida lipopolysaccharide. J Bacteriol. 1984 Apr;158(1):16–22. doi: 10.1128/jb.158.1.16-22.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann J., Wollum A. G. Simplified Enzyme-Linked Immunosorbent Assay for Routine Identification of Rhizobium japonicum Antigens. Appl Environ Microbiol. 1985 Apr;49(4):1010–1013. doi: 10.1128/aem.49.4.1010-1013.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak E. M., Urbano M. R., Dazzo F. B. Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind trifoliin A, a white clover lectin. J Bacteriol. 1981 Nov;148(2):697–711. doi: 10.1128/jb.148.2.697-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H., Yoshida T., Tanegashima C., Tanaka S. Improved direct method for determination of keto acids by 2,4-dinitrophenylhydrazine. Anal Biochem. 1971 Oct;43(2):349–356. doi: 10.1016/0003-2697(71)90263-6. [DOI] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., McGroarty E. J. High-molecular-weight components in lipopolysaccharides of Salmonella typhimurium, Salmonella minnesota, and Escherichia coli. J Bacteriol. 1985 May;162(2):738–745. doi: 10.1128/jb.162.2.738-745.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. M., Conrad H. E. Structural heterogeneity in the lipopolysaccharide of Salmonella newington. Arch Biochem Biophys. 1974 Jun;162(2):530–535. doi: 10.1016/0003-9861(74)90213-6. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]