Abstract
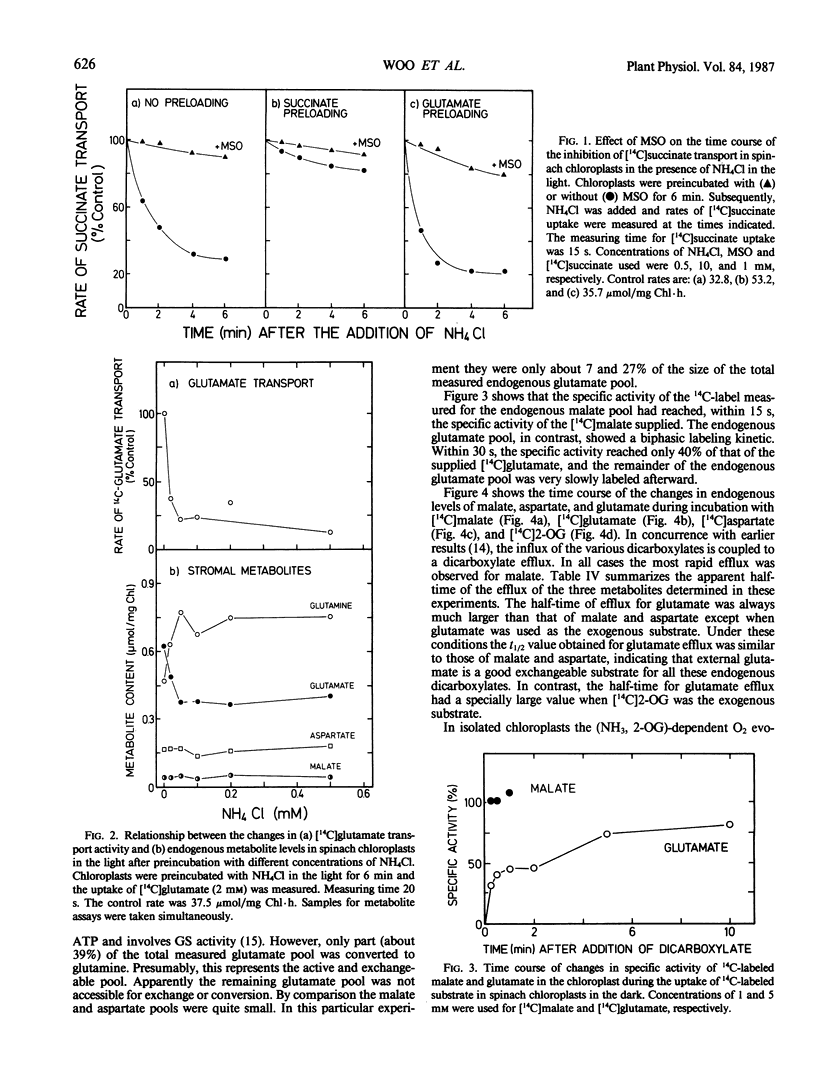
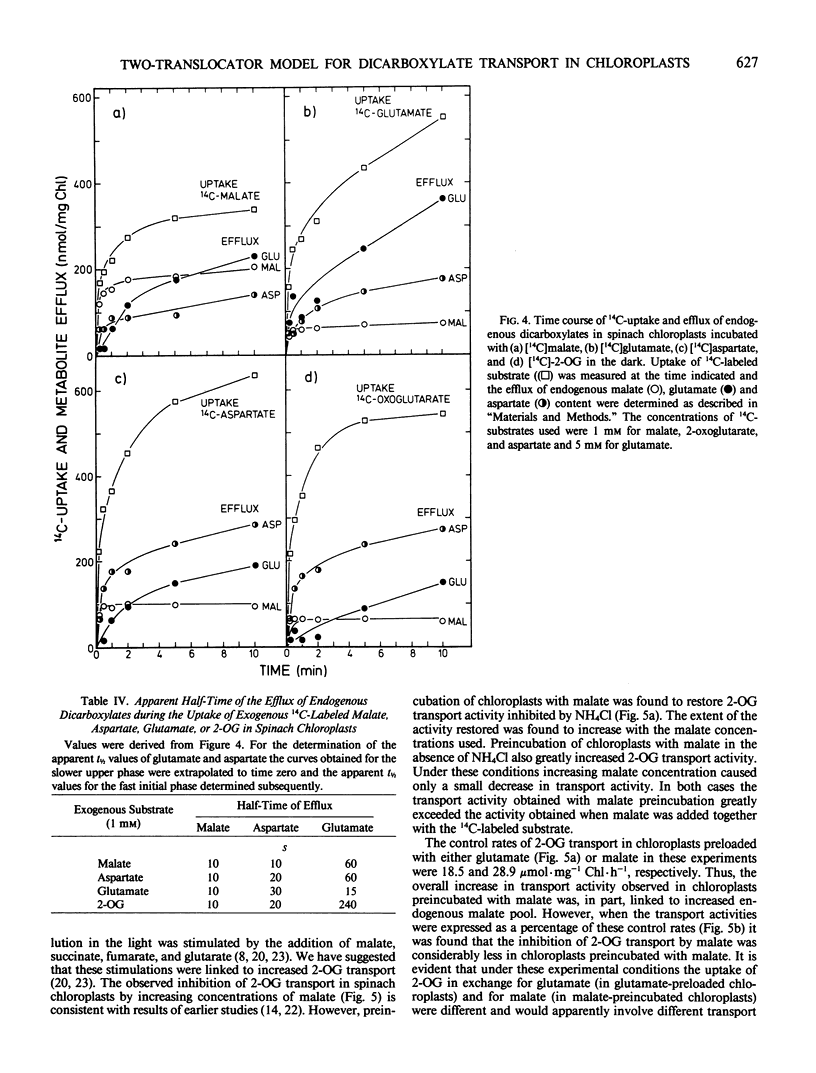
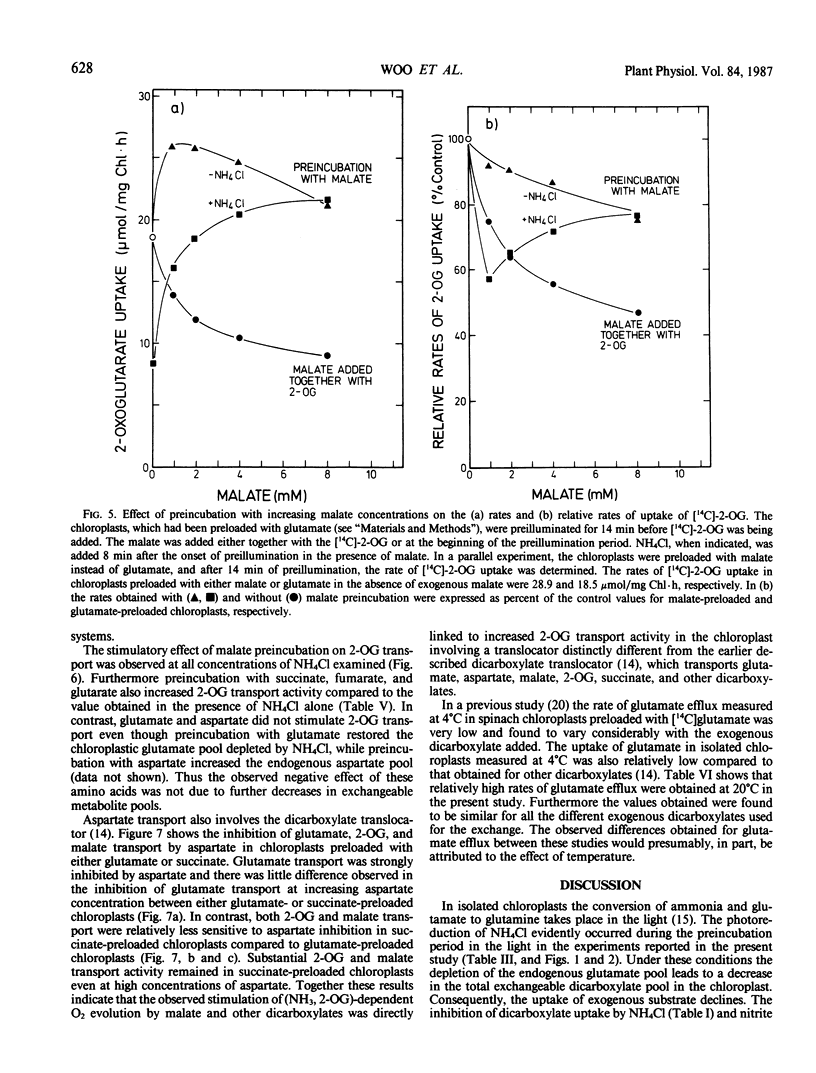
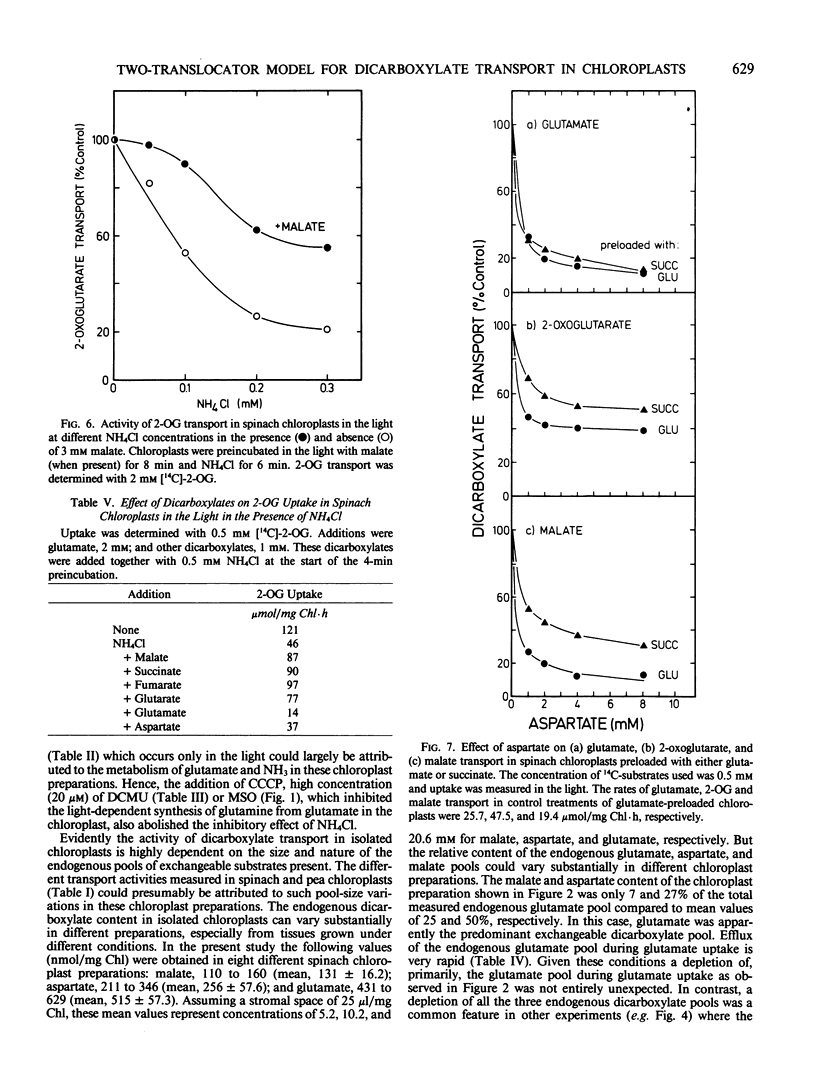
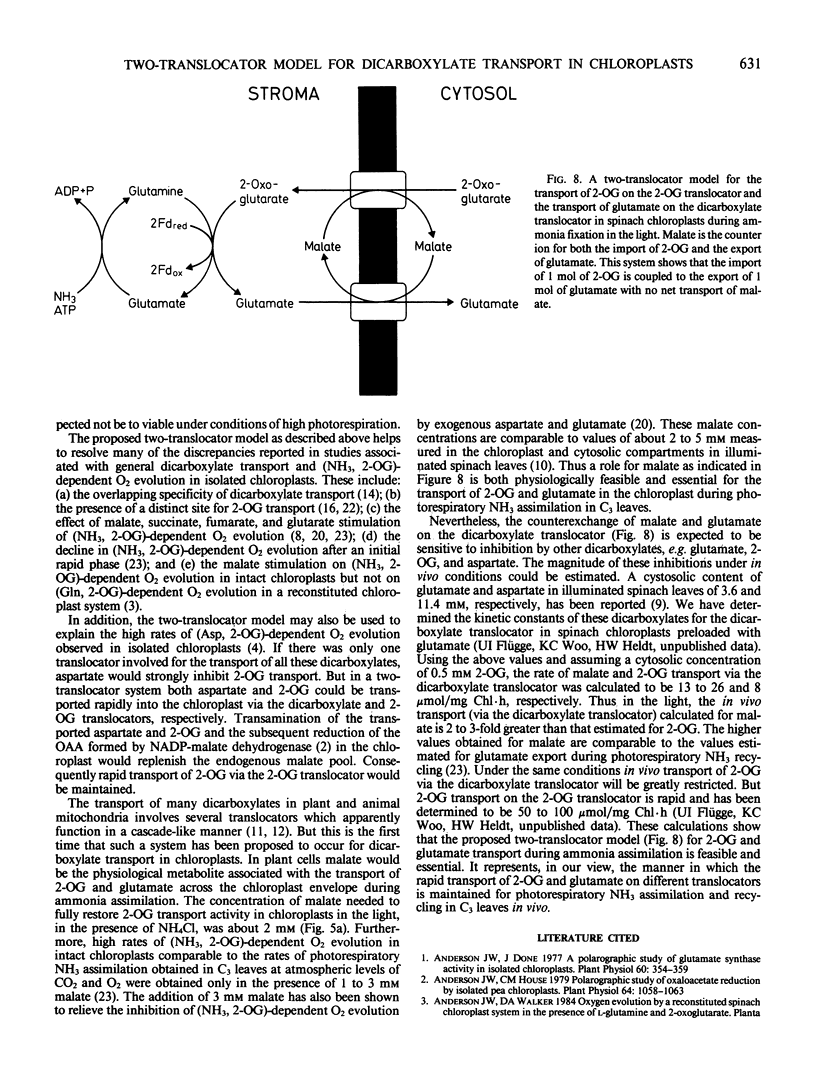
This study examines the transport of 2-oxoglutarate (2-OG) and other dicarboxylates during ammonia assimilation in illuminated spinach chloroplasts. The transport of all dicarboxylates examined was strongly inhibited by NH4Cl preincubation in the light. Treatment with NH4Cl caused a rapid depletion of the endogenous glutamate pool and a corresponding increase in endogenous glutamine content. The inhibition of transport activity by NH4Cl was apparently linked to its metabolism in the light because inhibition of glutamine synthetase activity by the addition of l-methionine sulfoximine or carbonylcyanide-m-chlorophenylhydrazone abolished this affect. Measurements of endogenous metabolite pools showed that malate was most rapidly exchanged during the uptake of all exogenous dicarboxylates examined. Depending on the exogenous substrates used, the apparent half-times of efflux measured for endogenous malate, aspartate and glutamate were 10, 10 to 30, and 15 to 240 seconds, respectively. The transport of 2-OG was also inhibited by malate. But chloroplasts preincubated with malate in the presence or absence of NH4Cl were found to have high transport activity similar to untreated chloroplasts. A two-translocator model is proposed to explain the stimulation of 2-OG transport as well as the stimulation of (NH3, 2-OG)-dependent O2 evolution by malate (KC Woo, CB Osmond 1982 Plant Physiol 69: 591-596) in isolated chloroplasts. In this model the transport of 2-OG on the 2-OG translocator and glutamate on the dicarboxylate translocator is coupled to malate counter-exchange in a cascade-like manner. This results in a net 2-OG/glutamate exchange with no net malate transport. Thus, during NH3 assimilation the transport of 2-OG into and the export of glutamate out of the chloroplast occurs via the 2-OG and the dicarboxylate translocators, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. A polarographic study of glutamate synthase activity in isolated chloroplasts. Plant Physiol. 1977 Sep;60(3):354–359. doi: 10.1104/pp.60.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., House C. M. Polarographic study of oxaloacetate reduction by isolated pea chloroplasts. Plant Physiol. 1979 Dec;64(6):1058–1063. doi: 10.1104/pp.64.6.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. The isolation of spinach chloroplasts in pyrophosphate media. Plant Physiol. 1968 Sep;43(9):1415–1418. doi: 10.1104/pp.43.9.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry I. B., Wiskich J. T. Characterization of dicarboxylate stimulation of ammonia, glutamine, and 2-oxoglutarate-dependent o(2) evolution in isolated pea chloroplasts. Plant Physiol. 1983 Jun;72(2):291–296. doi: 10.1104/pp.72.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Heldt H. W. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984 Jul;75(3):542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner K., Heldt H. W. Dicarboxylate transport across the inner membrane of the chloroplast envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):531–544. doi: 10.1016/0005-2728(78)90119-6. [DOI] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville S. C., Ogren W. L. An Arabidopsis thaliana mutant defective in chloroplast dicarboxylate transport. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1290–1294. doi: 10.1073/pnas.80.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Keegstra K. l-Aspartate Transport into Pea Chloroplasts : Kinetic and Inhibitor Evidence for Multiple Transport Systems. Plant Physiol. 1985 Jun;78(2):221–227. doi: 10.1104/pp.78.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C. Effect of inhibitors on ammonia-, 2-oxoglutarate-, and oxaloacetate-dependent o(2) evolution in illuminated chloroplasts. Plant Physiol. 1983 Jan;71(1):112–117. doi: 10.1104/pp.71.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Osmond C. B. Stimulation of ammonia and 2-oxoglutarate-dependent o(2) evolution in isolated chloroplasts by dicarboxylates and the role of the chloroplast in photorespiratory nitrogen recycling. Plant Physiol. 1982 Mar;69(3):591–596. doi: 10.1104/pp.69.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]


