Mechanistic probes for C–H amination and aziridination from (ArFL)Cu (4)a.
| Entry | Substrate | Product | Yield and commentary | Entry | Substrate | Product | Yield and commentary |
|---|---|---|---|---|---|---|---|
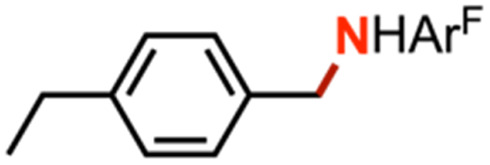
| 1b |
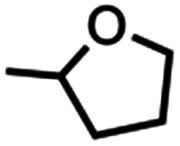
|
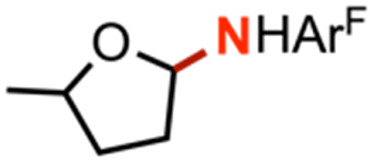
|
>99% 2° amination, no 3° amination | 4d |
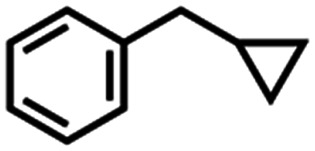
|
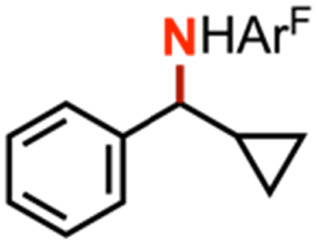
|
55(2)% no ring-opening |
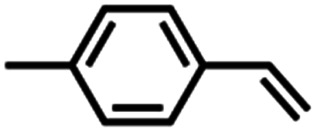
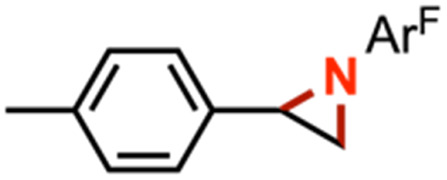
| 2c |
|
|
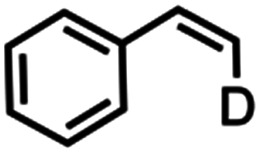
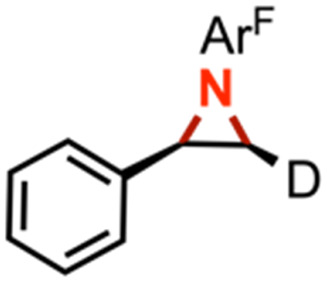
85(2)% aziridination, no amination | 5e,f |
|
|
85(1)% detection of only single diastereomer |
| 3b |
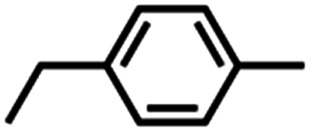
|
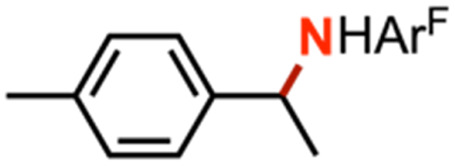
|
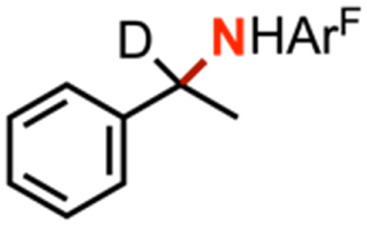
60(3)% enthalpy control 1.0(2°): 6.2(1°) | 6g |
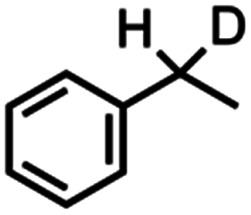
|
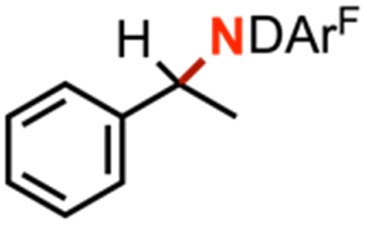
|
65(2)% sensitivity to H/D KIE 4.4(2) |
|
|
Yields determined by 19F NMR integration relative to fluorobenzene internal standard over a 16 h time frame from triplicate measurements in C6D6.
Neat substrate.
5 equiv. substrate.
30 equiv. substrate.
10 equiv. substrate.
Alternative diastereomer not observed by 1H NMR spectroscopy.
48 equiv. substrate. See ESI for reaction details.
