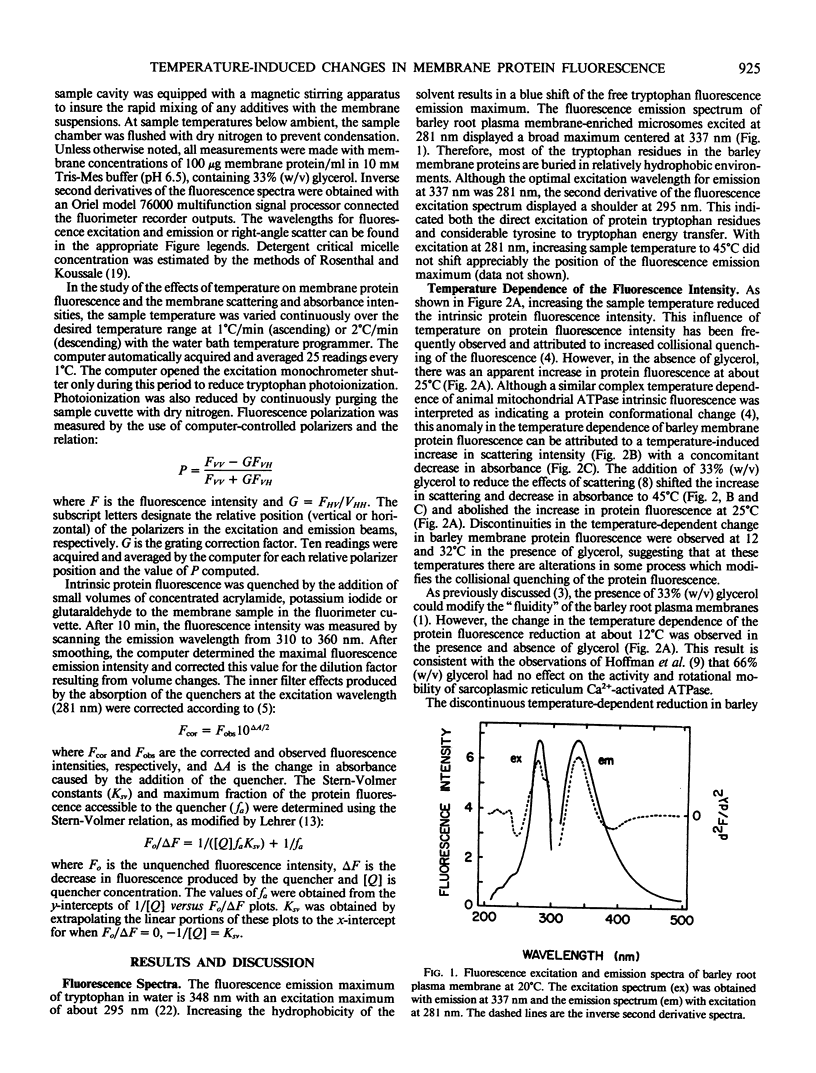
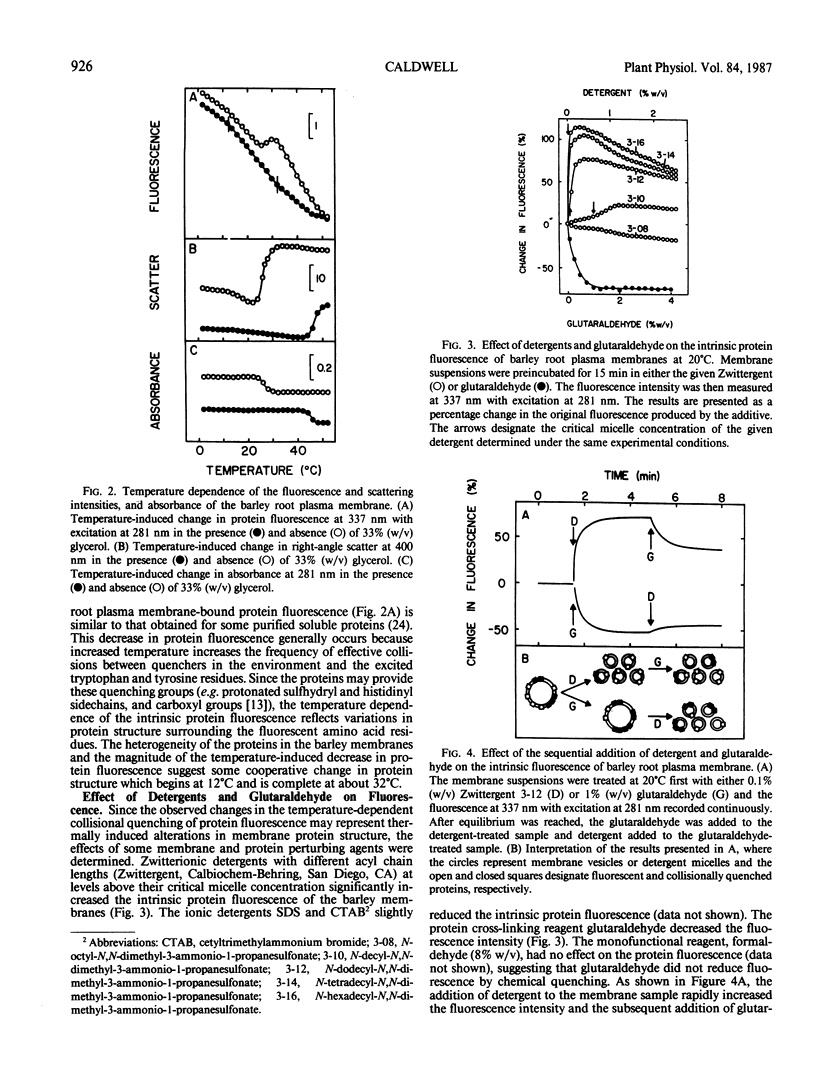
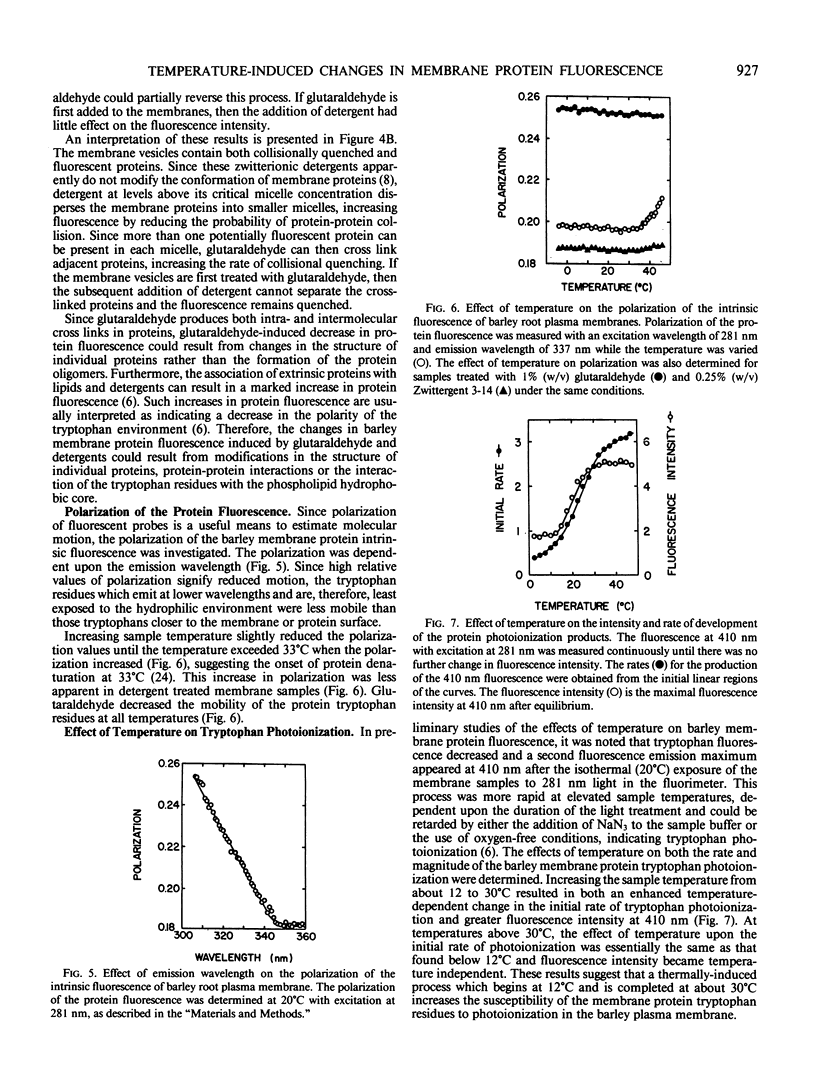
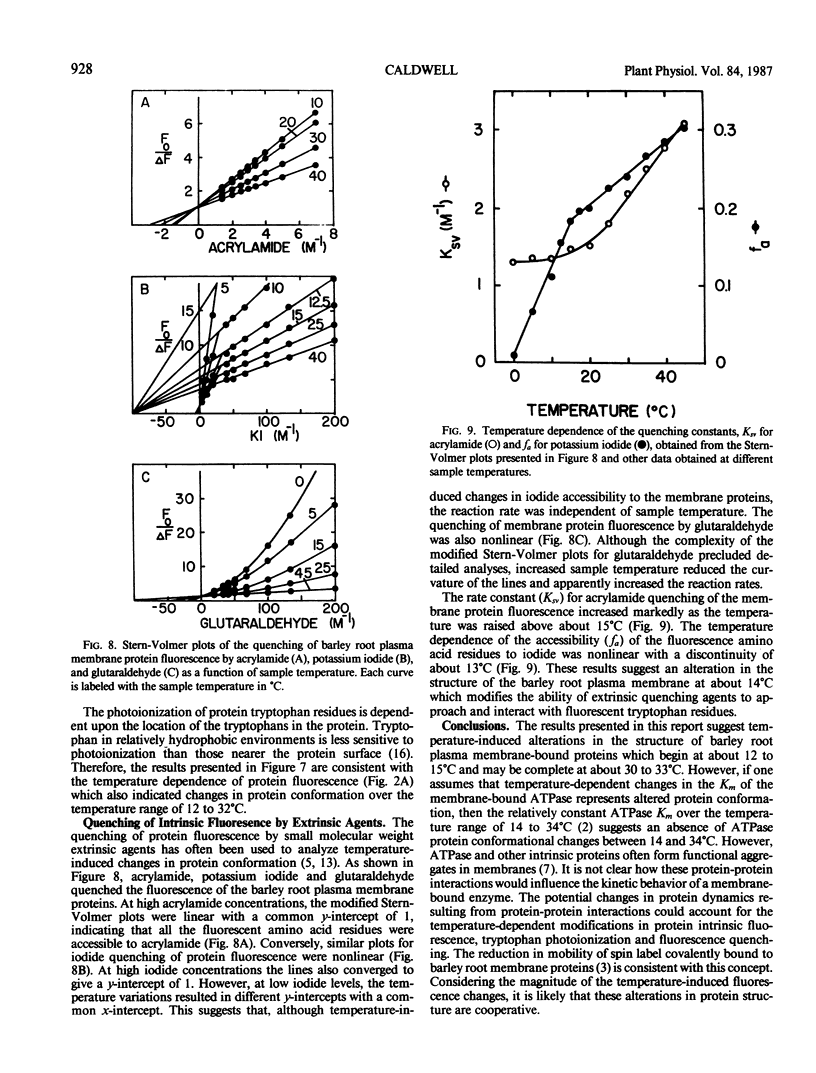
Abstract
The membrane-bound proteins of barley (Hordeum vulgare L. cv Conquest) root plasma membrane-enriched microsomes displayed fluorescence typical of protein-associated trytophan residues. The protein fluorescence intensity was sensitive to variations in sample temperature. The temperature-induced decline in protein fluorescence intensity was nonlinear with slope discontinuities at about 12 and 32°C. Detergents at levels above their critical micelle concentration enhanced protein fluorescence. Glutaraldehyde reduced protein fluorescence. Protein fluorescence polarization increased at temperatures above 30°C. Both the rate of tryptophan photoionization and the fluorescence intensity of the photoionization products suggested alterations in membrane protein conformation between 12 and 32°C. The quenching of the intrinsic protein fluorescence by acrylamide and potassium iodide indicated changes in accessibility of the extrinsic agents to the protein tryptophan residues beginning at about 14°C. The results indicate thermally induced changes in the dynamics of the membrane proteins over the temperature range of 12 to 32°C which could account for the complex temperature dependence of the barley root plasma membrane ATPase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett R. E. The effects of dimethylsulfoxide and glycerol on Na+, K+-ATPase and membrane structure. Cryobiology. 1978 Apr;15(2):227–229. doi: 10.1016/0011-2240(78)90029-9. [DOI] [PubMed] [Google Scholar]
- Caldwell C. R., Whitman C. E. Temperature-induced protein conformational changes in barley root plasma membrane-enriched microsomes: I. Effect of temperature on membrane protein and lipid mobility. Plant Physiol. 1987 Jul;84(3):918–923. doi: 10.1104/pp.84.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. G., Lee H. J. Monitoring protein conformational changes by quenching of intrinsic fluorescence. J Biochem Biophys Methods. 1984 Sep;9(4):351–355. doi: 10.1016/0165-022x(84)90019-8. [DOI] [PubMed] [Google Scholar]
- Devaux P. F., Seigneuret M. Specificity of lipid-protein interactions as determined by spectroscopic techniques. Biochim Biophys Acta. 1985 Jun 12;822(1):63–125. doi: 10.1016/0304-4157(85)90004-8. [DOI] [PubMed] [Google Scholar]
- Gomez-Fernandez J. C., Goni F. M., Bach D., Restall C. J., Chapman D. Protein-lipid interaction. Biophysical studies of (Ca2+ + Mg2+)-ATPase reconstituted systems. Biochim Biophys Acta. 1980 Jun 6;598(3):502–516. doi: 10.1016/0005-2736(80)90031-0. [DOI] [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann W., Sarzala M. G., Chapman D. Rotational motion and evidence for oligomeric structures of sarcoplasmic reticulum Ca2+-activated ATPase. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3860–3864. doi: 10.1073/pnas.76.8.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. G., Birdsall N. J., Metcalfe J. C., Toon P. A., Warren G. B. Clusters in lipid bilayers and the interpretation of thermal effects in biological membranes. Biochemistry. 1974 Aug 27;13(18):3699–3705. doi: 10.1021/bi00715a013. [DOI] [PubMed] [Google Scholar]
- Parenti Castelli G., Baracca A., Fato R., Rabbi A. A temperature-dependent structural change of mitochondrial ATPase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):366–372. doi: 10.1016/0006-291x(83)90315-7. [DOI] [PubMed] [Google Scholar]
- Pigault C., Gerard D. Influence of the location of tryptophanyl residues in proteins on their photosensitivity. Photochem Photobiol. 1984 Sep;40(3):291–297. doi: 10.1111/j.1751-1097.1984.tb04590.x. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Williams W. P. Plant lipids and their role in membrane function. Prog Biophys Mol Biol. 1978;34(2):109–173. doi: 10.1016/0079-6107(79)90016-6. [DOI] [PubMed] [Google Scholar]
- Raison J. K., Lyons J. M., Mehlhorn R. J., Keith A. D. Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem. 1971 Jun 25;246(12):4036–4040. [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Silvius J. R., McElhaney R. N. Non-linear Arrhenius plots and the analysis of reaction and motional rates in biological membranes. J Theor Biol. 1981 Jan 7;88(1):135–152. doi: 10.1016/0022-5193(81)90332-5. [DOI] [PubMed] [Google Scholar]
- TEALE F. W., WEBER G. Ultraviolet fluorescence of the aromatic amino acids. Biochem J. 1957 Mar;65(3):476–482. doi: 10.1042/bj0650476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]
- Wasylewski Z., Horowitz P. M. A fluorescence study of conformational changes induced by substrate and temperature in bovine liver thiosulfate sulfurtransferase. Biochim Biophys Acta. 1982 Feb 4;701(1):12–18. doi: 10.1016/0167-4838(82)90305-3. [DOI] [PubMed] [Google Scholar]


