Abstract
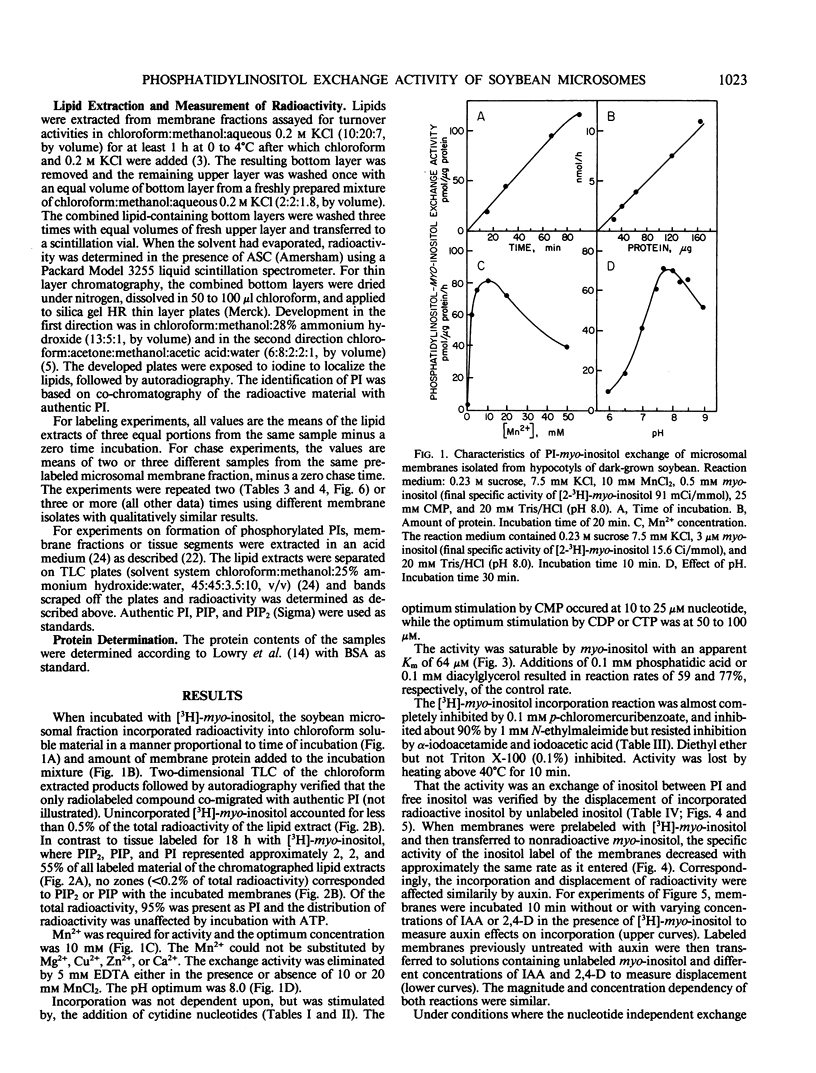
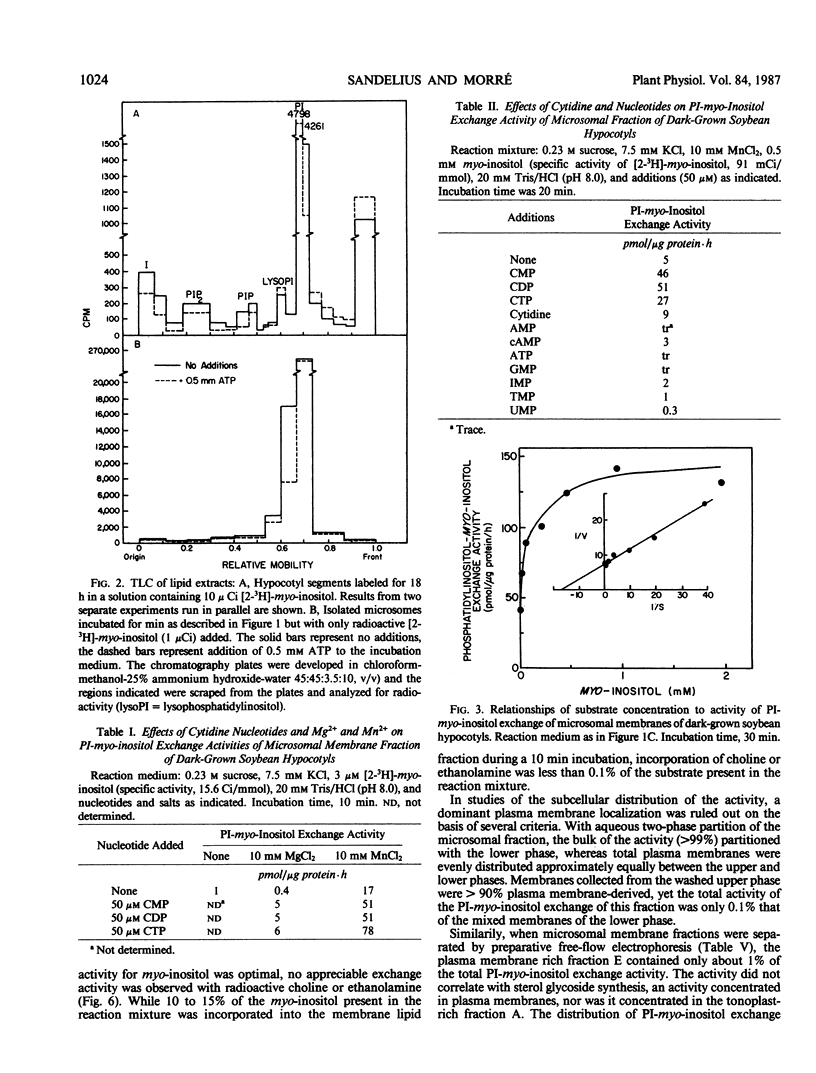
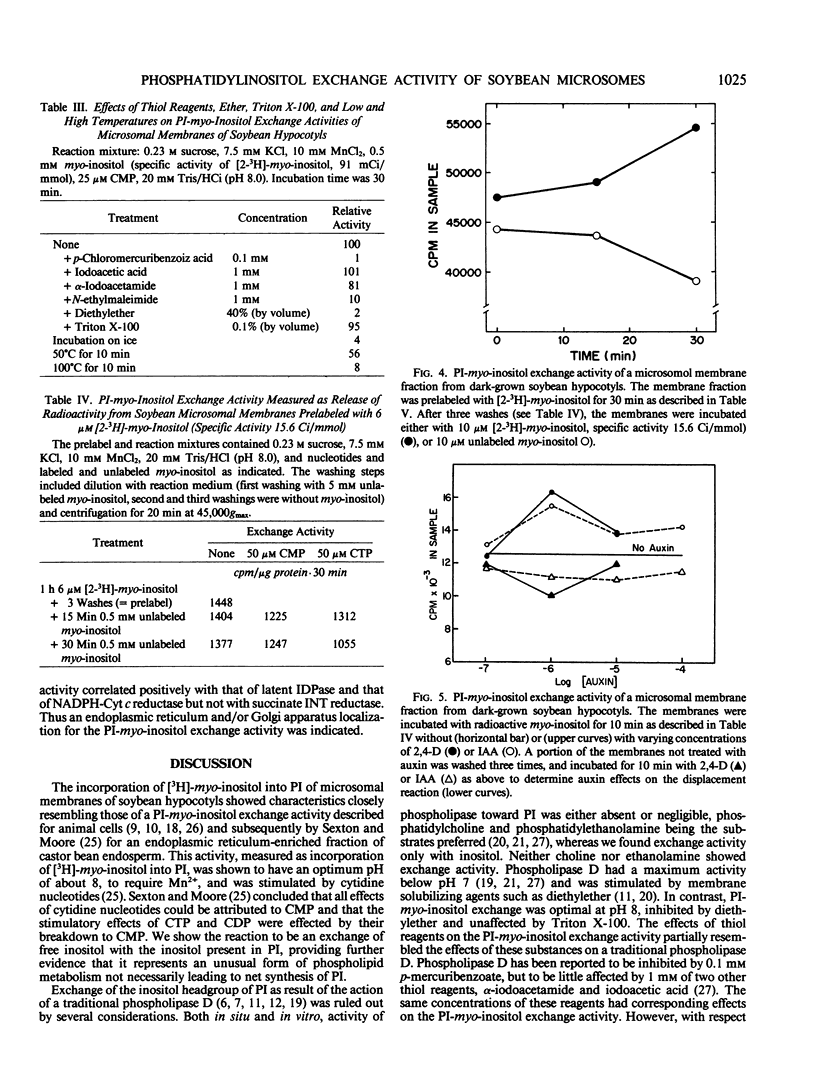
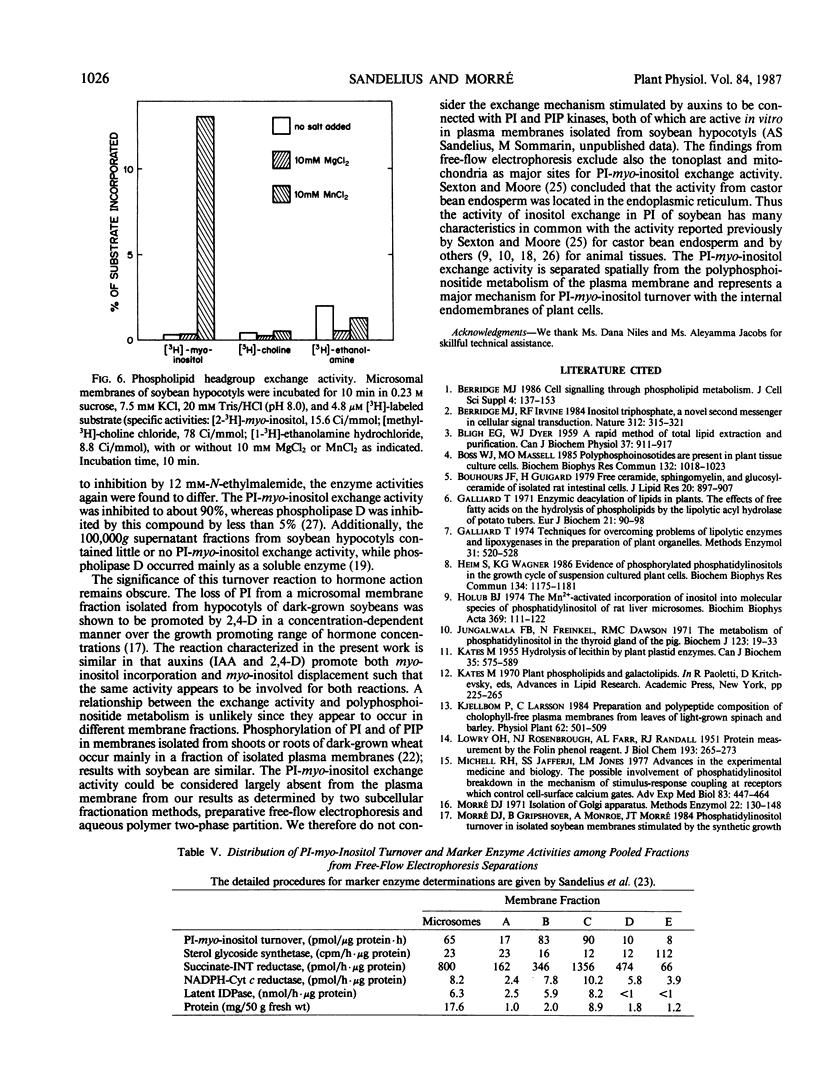
Microsome fractions from hypocotyls of dark-grown soybean (Glycine max [L.] Merrill) seedlings incorporated myo-inositol into phosphatidylinositol by an exchange reaction stimulated by Mn2+ (optimum at 10 mm) and cytidine nucleotides (CMP = CDP ≃ CTP) but not by Mg2+ or nucleotides other than cytidine nucleotides. The activity was membrane associated, with an optimum pH of 8, stimulated by auxin, and inhibited by certain thiol reagents or by heating above 40°C. With radioactive inositol, phosphatidylinositol was the only radioactive product. That turnover was by myo-inositol exchange was verified from experiments where unlabeled inositol replaced already incorporated inositol with approximately the same kinetics as for the incorporation of label. Both the incorporation and the displacement reactions were stimulated by Mn2+ and CMP and both were responsive to auxin with comparable dose dependency. Corresponding exchange activities with choline or ethanolamine were not observed. The phosphatidylinositol-myo-inositol exchange activity was low or absent from plasma membrane, tonoplast, and mitochondria enriched fractions. The activity co-localized on free-flow electrophoresis and aqueous two-phase partition with NADPH cytochrome c reductase and latent IDPase, markers for endoplasmic reticulum and Golgi apparatus, respectively. With microsomes incubated with both ATP and inositol, polyphosphoinositides were unlabeled demonstrating separate locations for the inositol exchange and phosphatidylinositol kinase reactions. Thus, the auxin-responsive inositol turnover activity of soybean membranes is distinct from the usual de novo biosynthetic pathway. It is not the result of a traditional D-type phospholipase and appears not to involve plasma membrane-associated polyphosphoinositide metabolism. It most closely resembles previously described phosphatidylinositol-myo-inositol exchange activities of plant and animal endoplasmic reticulum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Cell signalling through phospholipid metabolism. J Cell Sci Suppl. 1986;4:137–153. doi: 10.1242/jcs.1986.supplement_4.9. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Boss W. F., Massel M. O. Polyphosphoinositides are present in plant tissue culture cells. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1018–1023. doi: 10.1016/0006-291x(85)91908-4. [DOI] [PubMed] [Google Scholar]
- Bouhours J. F., Guignard H. Free ceramide, sphingomyelin, and glucosylceramide of isolated rat intestinal cells. J Lipid Res. 1979 Sep;20(7):879–907. [PubMed] [Google Scholar]
- Galliard T. Enzymic deacylation of lipids in plants. The effects of free fatty acids on the hydrolysis of phospholipids by the lipolytic acyl hydrolase of potato tubers. Eur J Biochem. 1971 Jul 15;21(1):90–98. doi: 10.1111/j.1432-1033.1971.tb01444.x. [DOI] [PubMed] [Google Scholar]
- Galliard T. Techniques for overcoming problems of lipolytic enzymes and lipoxygenases in the preparation of plant organelles. Methods Enzymol. 1974;31:520–528. doi: 10.1016/0076-6879(74)31056-7. [DOI] [PubMed] [Google Scholar]
- Heim S., Wagner K. G. Evidence of phosphorylated phosphatidylinositols in the growth cycle of suspension cultured plant cells. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1175–1181. doi: 10.1016/0006-291x(86)90374-8. [DOI] [PubMed] [Google Scholar]
- Holub B. J. The Mn2+-activated incorporation of inositol into molecular species of phosphatidylinositol in rat liver microsomes. Biochim Biophys Acta. 1974 Oct 16;369(1):111–122. doi: 10.1016/0005-2760(74)90197-0. [DOI] [PubMed] [Google Scholar]
- Jungalwala F. B., Freinkel N., Dawson R. M. The metabolism of phosphatidylinositol in the thyroid gland of the pig. Biochem J. 1971 Jun;123(1):19–33. doi: 10.1042/bj1230019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATES M. Hydrolysis of lecithin by plant plastid enzymes. Can J Biochem Physiol. 1955 Jul;33(4):575–589. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. The possible involvement of phosphatidylinositol breakdown in the mechanism of stimulus-response coupling at receptors which control cell-surface calcium gates. Adv Exp Med Biol. 1977;83:447–464. doi: 10.1007/978-1-4684-3276-3_41. [DOI] [PubMed] [Google Scholar]
- PAULUS H., KENNEDY E. P. The enzymatic synthesis of inositol monophosphatide. J Biol Chem. 1960 May;235:1303–1311. [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. The distribution of phospholipase D in developing and mature plants. Biochem J. 1969 May;112(5):787–794. doi: 10.1042/bj1120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Is phospholipase D really an enzyme? A comparison of in situ and in vitro activities. Biochim Biophys Acta. 1976 Apr 22;431(1):86–95. doi: 10.1016/0005-2760(76)90262-9. [DOI] [PubMed] [Google Scholar]
- Sandelius A. S., Penel C., Auderset G., Brightman A., Millard M., Morré D. J. Isolation of highly purified fractions of plasma membrane and tonoplast from the same homogenate of soybean hypocotyls by free-flow electrophoresis. Plant Physiol. 1986 May;81(1):177–185. doi: 10.1104/pp.81.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Sexton J. C., Moore T. S. Phosphatidylinositol synthesis by a mn-dependent exchange enzyme in castor bean endosperm. Plant Physiol. 1981 Jul;68(1):18–22. doi: 10.1104/pp.68.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F., Freer S., Benson A. A. Transphosphatidylation by phospholipase D. J Biol Chem. 1967 Feb 10;242(3):477–484. [PubMed] [Google Scholar]


