Abstract
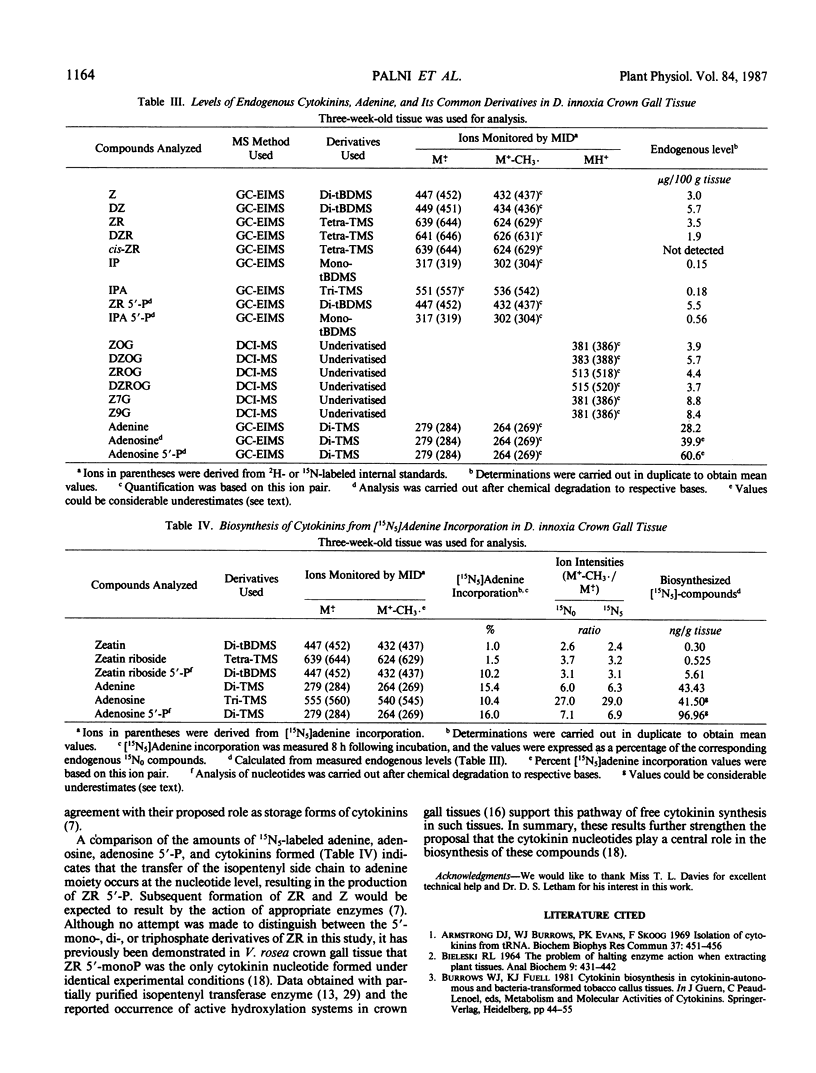
In this study gas chromatographic-mass spectrometric techniques have been used to identify and quantify the metabolic incorporation of [15N5]adenine into zeatin and its metabolites by 3-week-old Datura innoxia Mill, crown gall tissue. In a parallel study the levels of endogenous cytokinins were also determined by the stable isotope dilution technique using deuterium (2H)-labeled internal standards. Incorporation levels of the [15N5]adenine after 8 hours of incubation, expressed as a percentage of the endogenous cytokinins, were as follows: zeatin (1.0%), zeatin riboside (1.5%), and zeatin riboside 5′-phosphate (10.2%). These results are consistent with those observed in complementary experiments using [U-14C]adenine, and support the proposal that the cytokinin biosynthesis occurs primarily at the nucleotide level. The effect of tissue age on cytokinin biosynthesis, determined by [U-14C]adenine incorporation into cytokinins by tissues at varying growth stages, indicated a steady increase with time reaching maximal synthesis at five weeks following subculture after which the level of 14C incorporation into cytokinins declined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. J., Burrows W. J., Evans P. K., Skoog F. Isolation of cytokinins from tRNA. Biochem Biophys Res Commun. 1969 Oct 22;37(3):451–456. doi: 10.1016/0006-291x(69)90936-x. [DOI] [PubMed] [Google Scholar]
- BIELESKI R. L. THE PROBLEM OF HALTING ENZYME ACTION WHEN EXTRACTING PLANT TISSUES. Anal Biochem. 1964 Dec;9:431–442. doi: 10.1016/0003-2697(64)90204-0. [DOI] [PubMed] [Google Scholar]
- Gamborg O. L., Eveleigh D. E. Culture methods and detection of glucanases in suspension cultures of wheat and barley. Can J Biochem. 1968 May;46(5):417–421. doi: 10.1139/o68-063. [DOI] [PubMed] [Google Scholar]
- Miller C. O. Cell-division factors from Vinca rosca L. crown gall tumor tissue. Proc Natl Acad Sci U S A. 1975 May;72(5):1883–1886. doi: 10.1073/pnas.72.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Ribosyl-trans-Zeatin, A Major Cytokinin Produced by Crown Gall Tumor Tissue. Proc Natl Acad Sci U S A. 1974 Feb;71(2):334–338. doi: 10.1073/pnas.71.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. O. Mass Spectroscopic Identification of Cytokinins: Glucosyl Zeatin and Glucosyl Ribosylzeatin from Vinca rosea Crown Gall. Plant Physiol. 1977 Jun;59(6):1029–1033. doi: 10.1104/pp.59.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palni L. M., Summons R. E., Letham D. S. Mass spectrometric analysis of cytokinins in plant tissues : v. Identification of the cytokinin complex of datura innoxia crown gall tissue. Plant Physiol. 1983 Jul;72(3):858–863. doi: 10.1104/pp.72.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. B., Miller C. O. Glucosyl Zeatin and Glucosyl Ribosylzeatin from Vinca rosea L. Crown Gall Tumor Tissue. Plant Physiol. 1977 Jun;59(6):1026–1028. doi: 10.1104/pp.59.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton K. R., Clark J. M., Jr A proton exchange between purines and water and its application to biochemistry. Biochemistry. 1967 Sep;6(9):2735–2739. doi: 10.1021/bi00861a013. [DOI] [PubMed] [Google Scholar]
- Taya Y., Tanaka Y., Nishimura S. 5'-AMP is a direct precursor of cytokinin in Dictyostelium discoideum. Nature. 1978 Feb 9;271(5645):545–547. doi: 10.1038/271545a0. [DOI] [PubMed] [Google Scholar]


