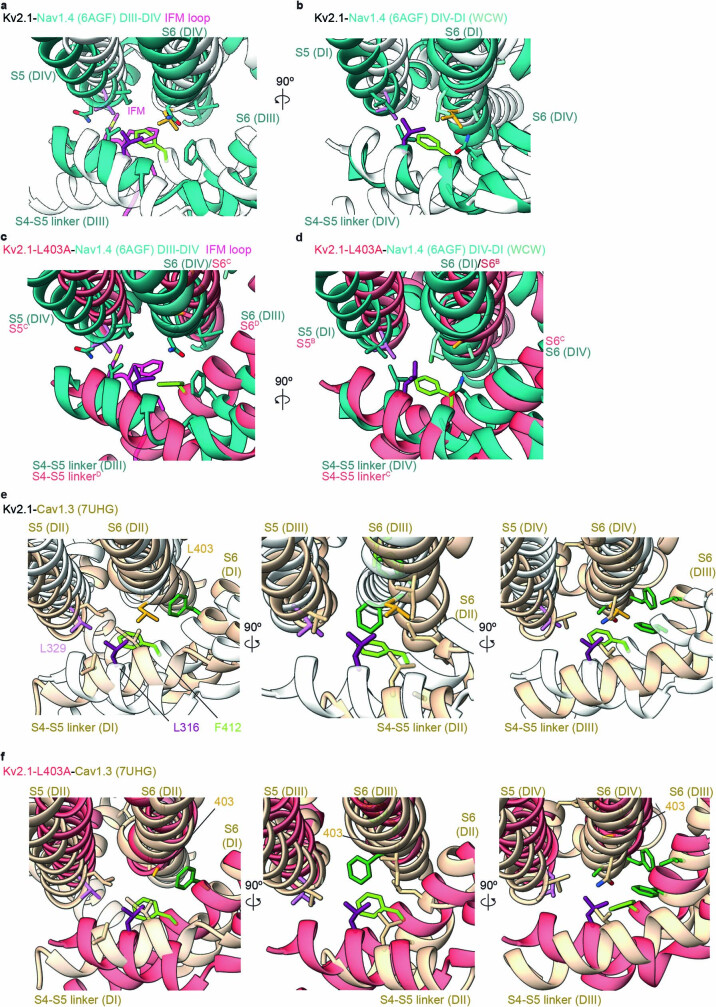
Extended Data Fig. 10. Structural alignment of Kv2.1 with Nav1.4 and Cav1.3 channels.
a) Close-up view of the hydrophobic coupling nexus residues in Kv2.1 (white) and between domains III and IV of Nav1.4 (blue). b) Hydrophobic coupling nexus residues in Kv2.1 (white) and between domains IV and I of Nav1.4 (blue). WCW mutants (see Discussion) are shown in stick representation (light blue). c) Hydrophobic coupling nexus residues in the L403A mutant of Kv2.1 (red) and between domains III and IV of Nav1.4 (blue). d) Hydrophobic coupling nexus residues in the L403A mutant of Kv2.1 (red) and between domains IV and I of Nav1.4 (blue). e,f) Close-up view of the hydrophobic coupling nexus residues highlighted with the side chains depicted as sticks with Kv2.1 residues labeled for Kv2.1 (white) and Cav1.3 (tan) (e) or Kv2.1-L403A (red) and Cav1.3 (tan) (f). Dark green side chains identify residues where mutations enhance voltage dependent inactivation59.