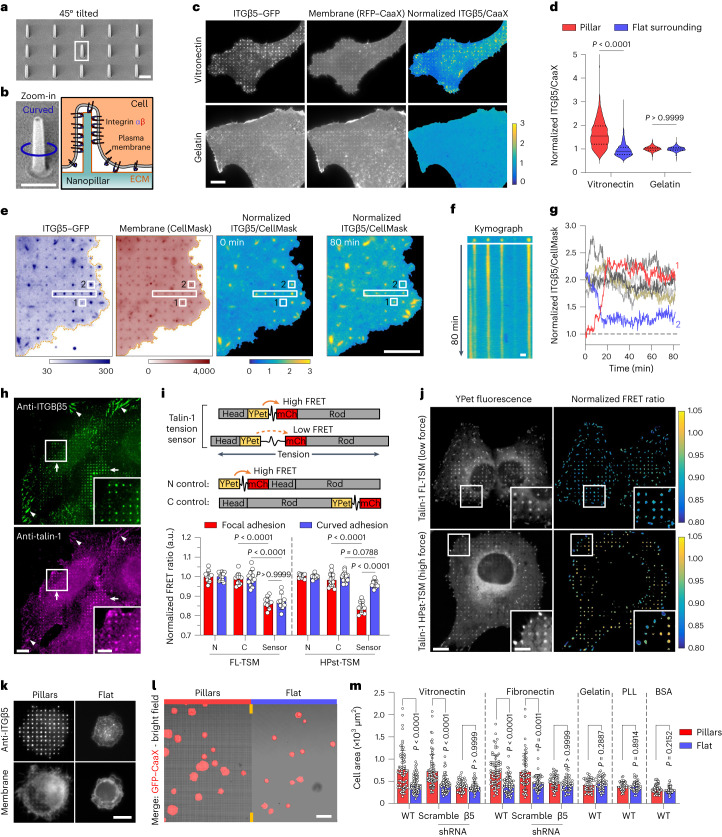
Fig. 2. Curved adhesions recruit talin-1 and bear low mechanical forces.
a, An SEM image of nanopillars. Scale bar, 1 µm. b, Left: zoom-in on a single nanopillar in a. Scale bar, 1 µm. Right: Schematic of integrin adhesion at a nanopillar. c, Fluorescence images showing that vitronectin-coated but not gelatin-coated nanopillars induce ITGβ5–GFP accumulation relative to RFP–CaaX. Ratiometric images are shown in the Parula colour scale. Scale bar, 10 µm. d, Quantifications of the normalized ITGβ5/CaaX ratio at individual nanopillars and their flat surrounding regions. Left to right, n = 926, 926, 863 and 863 pillars, from 3 independent cells. Medians (lines) and quartiles (dotted lines) are shown. e, Live-cell imaging of ITGβ5–GFP with CellMask membrane marker on vitronectin-coated nanopillars at 15 s per frame for 80 min. Ratiometric images at 0 min and 80 min are shown. Scale bar, 10 µm. f, Kymograph of the rectangular box in e. Scale bar, 1 µm. g, Example trajectories of ITGβ5 nanopillar accumulations showing that most curved adhesions persist, with a few that slowly assemble (pillar 1) or disassemble (pillar 2). h, On vitronectin-coated substrates, endogenous ITGβ5 and talin-1 colocalize in both curved adhesions at nanopillars (arrows) and focal adhesions between nanopillars on flat areas (arrowheads). Scale bar, 10 µm (full size) or 5 µm (insets). i, Top: schematic of talin-1 tension sensors. mCh, mCherry. Bottom: quantification of the normalized average FRET ratio (in arbitrary units (a.u.)) at focal adhesions and curved adhesions. Left to right, n = 13, 12, 10, 10, 15 and 11 cells, from 2 independent experiments. j, Fluorescence (YPet) and ratiometric FRET images (in the Parula colour scale) of the low-force FL-TSM sensor and the high-force HPst-TSM sensor. In the zoom-in window of the HPst-TSM sensor, curved adhesions exhibit higher FRET values, and thus lower tensions, than focal adhesions. Scale bar, 10 µm (full size) or 5 µm (insets). k, ITGβ5 accumulates at vitronectin-coated nanopillars within 30 min after seeding. Scale bar, 10 µm. l, Early-stage cell spreading (30 min after plating) on vitronectin-coated nanopillar and flat areas in the same image. Scale bar, 50 µm. m, Quantification of the cell spreading area. n are as follows (left to right): n = 69, 68, 79, 53, 45 and 48 (vitronectin) and n = 76, 64, 57, 55, 45 and 42 (fibronectin) cells, from 3 independent experiments; n = 38 and 44 (gelatin), n = 35 and 38 (PLL) and n = 42 and 34 (BSA) cells, from 2 independent experiments. Data are the mean ± s.d. (i,m). P values calculated using Kruskal–Wallis test with Dunn’s multiple comparison (d,m (vitronectin, fibronectin)), one-way ANOVA with Tukey’s multiple comparison (i), Mann–Whitney test (m, gelatin) or two-tailed t-test (m, PLL, BSA). Source numerical data are available in the source data.