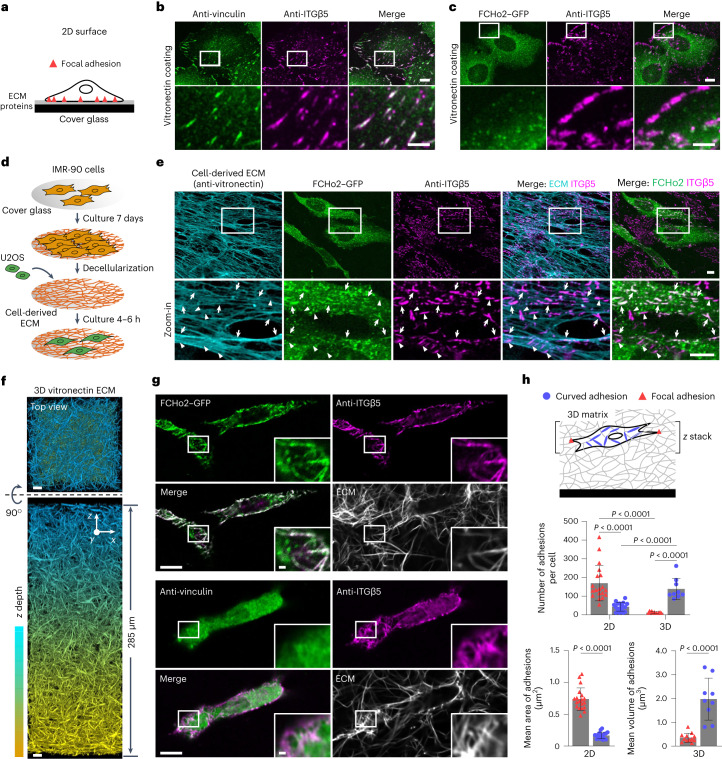
Fig. 5. Curved adhesions are prevalent in physiologically relevant environments.
a, Schematic of a cell growing on ECM-coated 2D flat surfaces. b, ITGβ5 and vinculin strongly colocalize on vitronectin-coated flat surfaces. Scale bar, 10 µm (full size) or 5 µm (insets). c, ITGβ5 and FCHo2 do not colocalize on vitronectin-coated flat surfaces. Scale bar, 10 µm (full size) or 5 µm (insets). d, Schematic illustrating the generation of cell-derived ECM fibres. e, IMR-90-derived ECM fibres have vitronectin incorporated as shown by anti-vitronectin staining. U2OS cells form both curved adhesions (arrows, colocalization of anti-ITGB5 with FCHo2–GFP) and focal adhesions (arrowheads, anti-ITGB5 devoid of FCHo2–GFP) on these fibres. Scale bar, 10 µm. f, Representative 3D images of a thick layer of matrices made of vitronectin fibres. Scale bar, 10 µm. g, Curved adhesions, marked by the colocalizations of ITGβ5 and FCHo2, are abundant on vitronectin fibres in 3D matrices (top), whereas focal adhesions marked by the colocalization of ITGβ5 and vinculin are sparse (bottom). Scale bar, 10 µm (full size) or 1 µm (insets). h, Quantification of the number and size of focal adhesions and curved adhesions on 2D flat surfaces and in 3D matrices of vitronectin fibres. Left to right, n = 19, 18, 12 and 9 cells, from 2 independent experiments. Data are the mean ± s.d. P values calculated using two-tailed t-test (h, adhesion size) or Kruskal–Wallis test with Dunn’s multiple-comparison (h, adhesion number). Source numerical data are available in the source data.