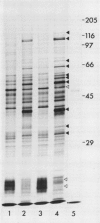
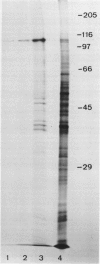
Abstract
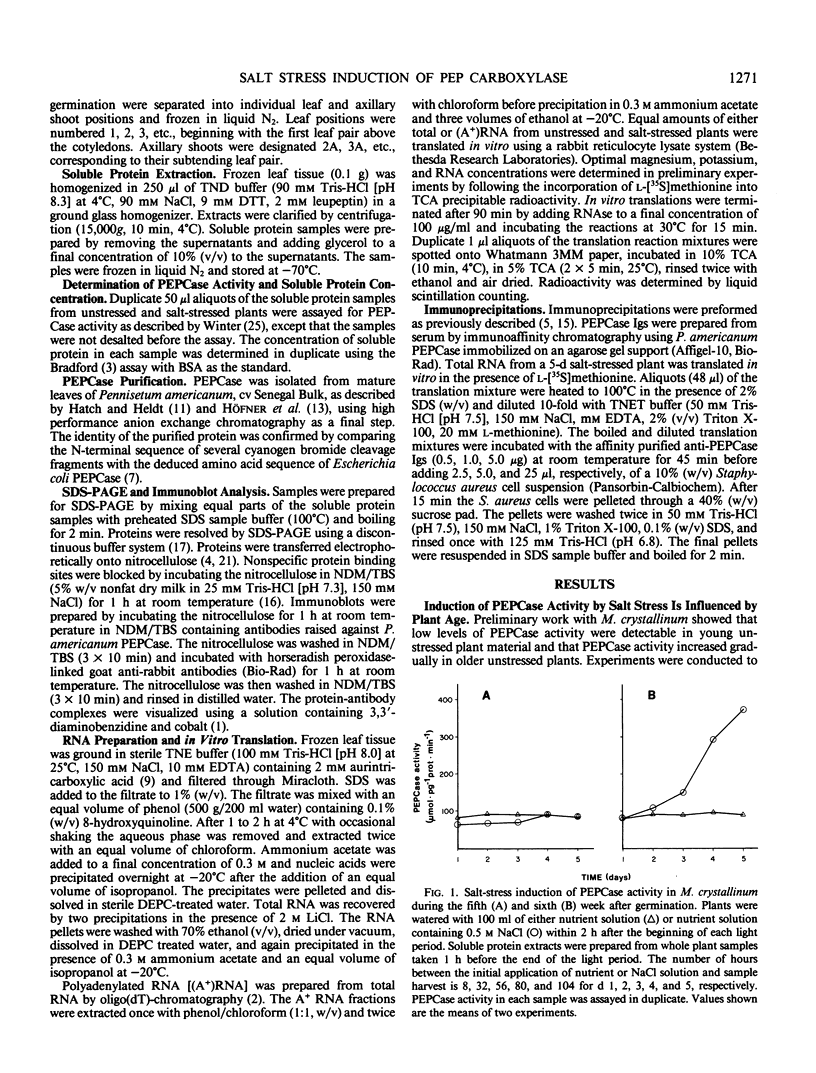
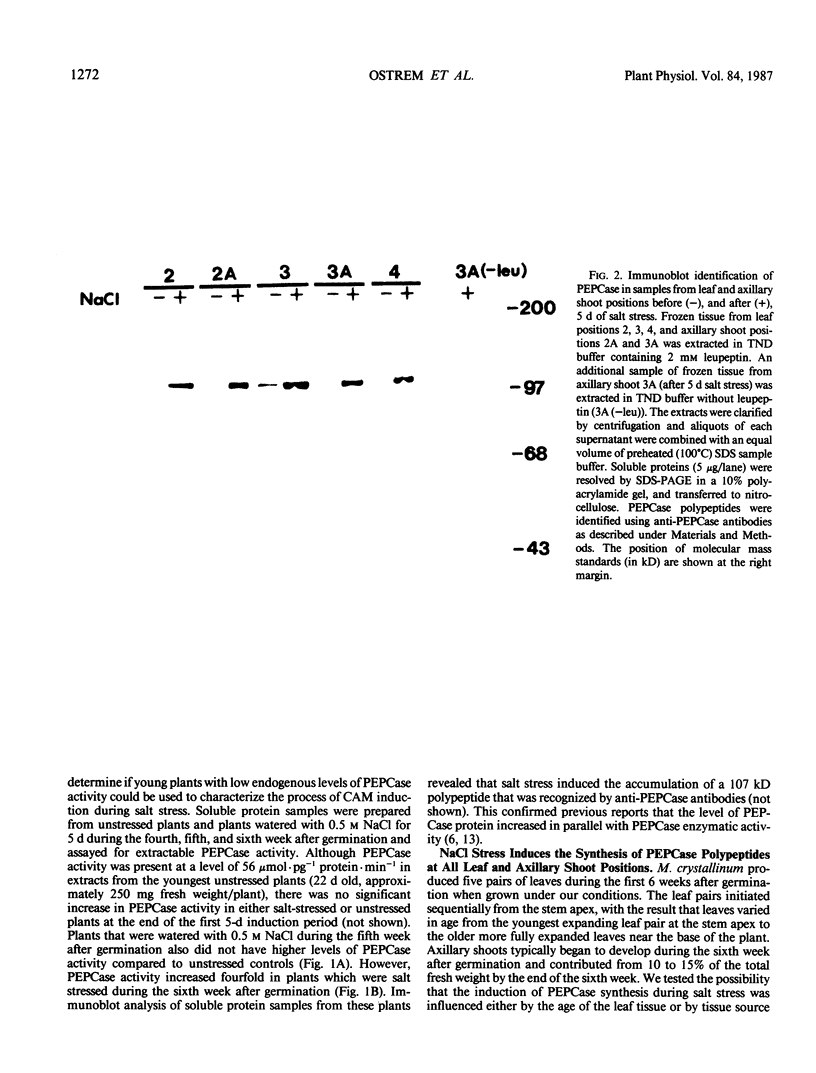
Mesembryanthemum crystallinum responds to salt stress by switching from C3 photosynthesis to Crassulacean acid metabolism (CAM). During this transition the activity of phosphoenolpyruvate carboxylase (PEPCase) increases in soluble protein extracts from leaf tissue. We monitored CAM induction in plants irrigated with 0.5 molar NaCl for 5 days during the fourth, fifth, and sixth week after germination. Our results indicate that the age of the plant influenced the response to salt stress. There was no increase in PEPCase protein or PEPCase enzyme activity when plants were irrigated with 0.5 molar NaCl during the fourth and fifth week after germination. However, PEPCase activity increased within 2 to 3 days when plants were salt stressed during the sixth week after germination. Immunoblot analysis with anti-PEPCase antibodies showed that PEPCase synthesis was induced in both expanded leaves and in newly developing axillary shoot tissue. The increase in PEPCase protein was paralleled by an increase in PEPCase mRNA as assayed by immunoprecipitation of PEPCase from the in vitro translation products of RNA from salt-stressed plants. These results demonstrate that salinity increased the level of PEPCase in leaf and shoot tissue via a stress-induced increase in the steady-state level of translatable mRNA for this enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Colbert J. T., Hershey H. P., Quail P. H. Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2248–2252. doi: 10.1073/pnas.80.8.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N., Miwa T., Ishijima S., Izui K., Katsuki H. The primary structure of phosphoenolpyruvate carboxylase of Escherichia coli. Nucleotide sequence of the ppc gene and deduced amino acid sequence. J Biochem. 1984 Apr;95(4):909–916. doi: 10.1093/oxfordjournals.jbchem.a134718. [DOI] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpster M. H., Taylor W. C. Maize phosphoenolpyruvate carboxylase. Cloning and characterization of mRNAs encoding isozymic forms. J Biol Chem. 1986 May 5;261(13):6132–6136. [PubMed] [Google Scholar]
- Hatch M. D., Heldt H. W. Synthesis, storage, and stability of [4-14C]oxaloacetic acid. Anal Biochem. 1985 Mar;145(2):393–397. doi: 10.1016/0003-2697(85)90379-3. [DOI] [PubMed] [Google Scholar]
- Höfner R., Vazquez-Moreno L., Winter K., Bohnert H. J., Schmitt J. M. Induction of Crassulacean Acid Metabolism in Mesembryanthemum crystallinum by High Salinity: Mass Increase and de Novo Synthesis of PEP-Carboxylase. Plant Physiol. 1987 Apr;83(4):915–919. doi: 10.1104/pp.83.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K., Foster J. G., Edwards G. E., Holtum J. A. Intracellular Localization of Enzymes of Carbon Metabolism in Mesembryanthemum crystallinum Exhibiting C(3) Photosynthetic Characteristics or Performing Crassulacean Acid Metabolism. Plant Physiol. 1982 Feb;69(2):300–307. doi: 10.1104/pp.69.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]