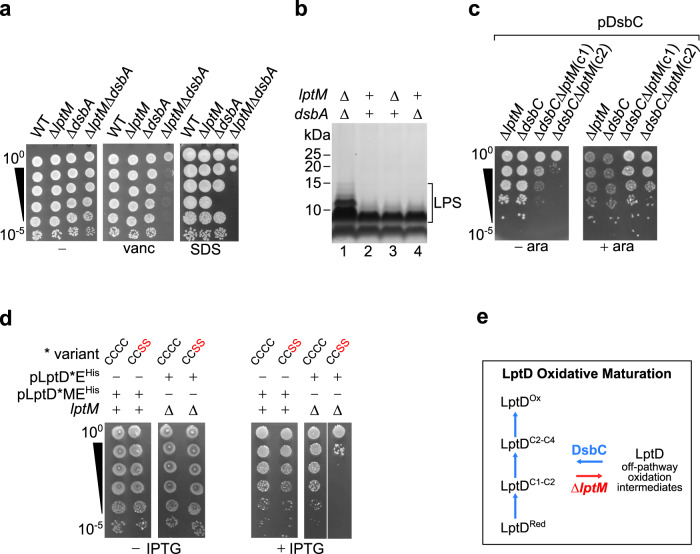
Fig. 4. LptM functions synergistically with the oxidative folding machinery.
a Drop dilution growth test of E. coli wild-type and the indicated derivative strains lacking lptM, dsbA or both under different conditions as indicated. vanc, vancomycin; SDS, sodium dodecyl sulfate. b LPS was extracted from the indicated strains, resolved by SDS-PAGE and silver stained. Results are representative of four independent experiments. c Drop dilution growth test of the indicated derivative strains lacking lptM, dsbC or both transformed with pDsbC on media lacking or supplemented with 0.02% arabinose (ara). d Drop dilution growth test of lptM+ or ΔlptM strains transformed with the indicated plasmids pLptD*EHis or pLptD*MEHis on media lacking or supplemented with 100 μM IPTG. * indicates variants of plasmid-encoded LptD, the wild-type LptDCCCC or the LptDCCSS variant, as specified on the top of each dilution test. e Schematic representation of the LptD oxidative maturation pathway. In wild-type cells the four cysteines of LptD are oxidized stepwise: LptDRed is first oxidized to form LptDC1-C2. Disulfide bond shuffling in the latter generates LptDC2-C4. A final event of oxidation forms a disulfide between C1 and C3, thus generating LptDOx. LptM acts synergistically with the disulfide bond formation machinery in facilitating proper oxidation of LptD. In the absence of LptM (ΔlptM, red arrow), a considerable fraction of LptD accumulates as off-pathway oxidation intermediates, most prominently LptDC1-C2. In this mutant, the assembly of LptD together with LptE stalls at the BAM complex and DsbC becomes essential for cell viability, suggesting that disulfide bond isomerization helps to rescue LPS translocon assembly.