Table 1.
Optimization of the reaction conditions
| Entry | Catalyst (x mol%) | Solvent | Yield (%)a | |
|---|---|---|---|---|
| 2a | 3a | |||
| 1b | ZnCl2 (20.0) | DCM | <5 | 6 |
| 2c | AgSbF6 (5.0) | DCM | – | – |
| 3c | JohnPhos(MeCN)AuSbF6 (5.0) | DCM | – | – |
| 4 | Cu(MeCN)4PF6 (5.0) | DCM | 15 | 30 |
| 5 | [PdCl(η3-C3H5)]2 (5.0) | DCM | 21 | <5 |
| 6 | Rh2(OAc)4 (2.0) | DCM | 44 | 20 |
| 7 | Rh2(Oct)4 (2.0) | DCM | 45 | 19 |
| 8 | Rh2(TPA)4 (2.0) | DCM | 41 | 15 |
| 9 | Rh2(OPiv)4 (2.0) | DCM | 54 | 10 |
| 10 | Rh2(esp)2 (2.0) | DCM | 43 | 9 |
| 11 | Rh2(pfb)4 (2.0) | DCM | 20 | 17 |
| 12 | Rh2(TFA)4 (2.0) | DCM | 16 | 14 |
| 13 | Rh2(tfacam)4 (2.0) | DCM | 20 | 65 |
| 14 | Rh2(cap)4 (2.0) | DCM | NR | NR |
| 15 | Rh2(OPiv)4 (2.0) | DCE | 74 | 13 |
| 16 | Rh2(OPiv)4 (2.0) | toluene | 47 | 12 |
| 17 | Rh2(OPiv)4 (2.0) | CH3CN | 71 | 10 |
| 18 | Rh2(OPiv)4 (2.0) | EtOAc | 81 | <5 |
| 19 | Rh2(OAc)4 (2.0) | EtOAc | 80 | <5 |
| 20 | Rh2(OPiv)4 (1.0) | EtOAc | 83 | <5 |
| 21d | Rh2(OPiv)4 (1.0) | EtOAc | 88 (84)e | <5 |
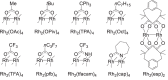 | ||||
Reaction conditions: to a solution of metal catalyst (x mol%) in 1.0 mL solvent, was added 1a (0.1 mmol, 34.4 mg) in the same solvent (1.0 mL) slowly over 10 min via a syringe at 40 °C under an argon atmosphere, and the reaction mixture was stirred for additional 1.0 h under these conditions.
DCM dichloromethane, DCE dichloroethane, EtOAc ethyl acetate.
aYields were detected by proton NMR of the crude reaction mixture using 4-nitrobenzaldehyde as the internal standard.
bLow conversions (<20%) were observed, and most of the material was recovered.
cThe material 1a was decomposed into a complex mixture.
d100 mg 4 Å MS was added.
eIsolated yield.
