Abstract
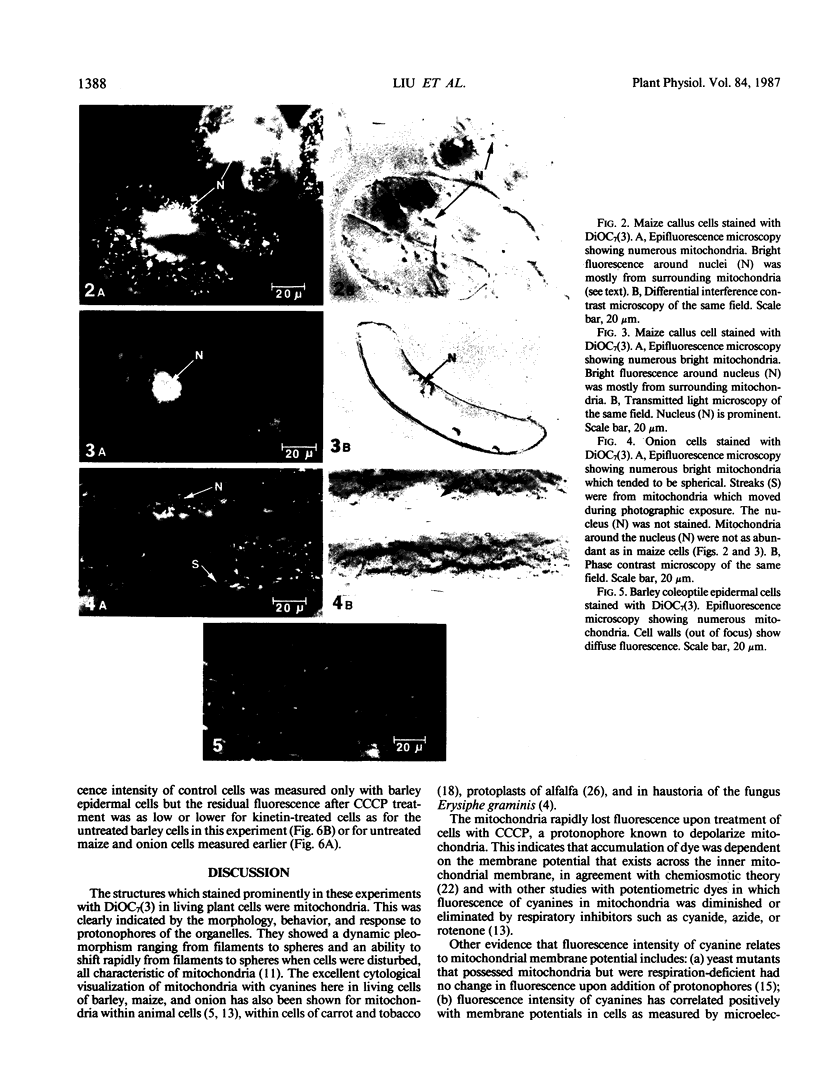
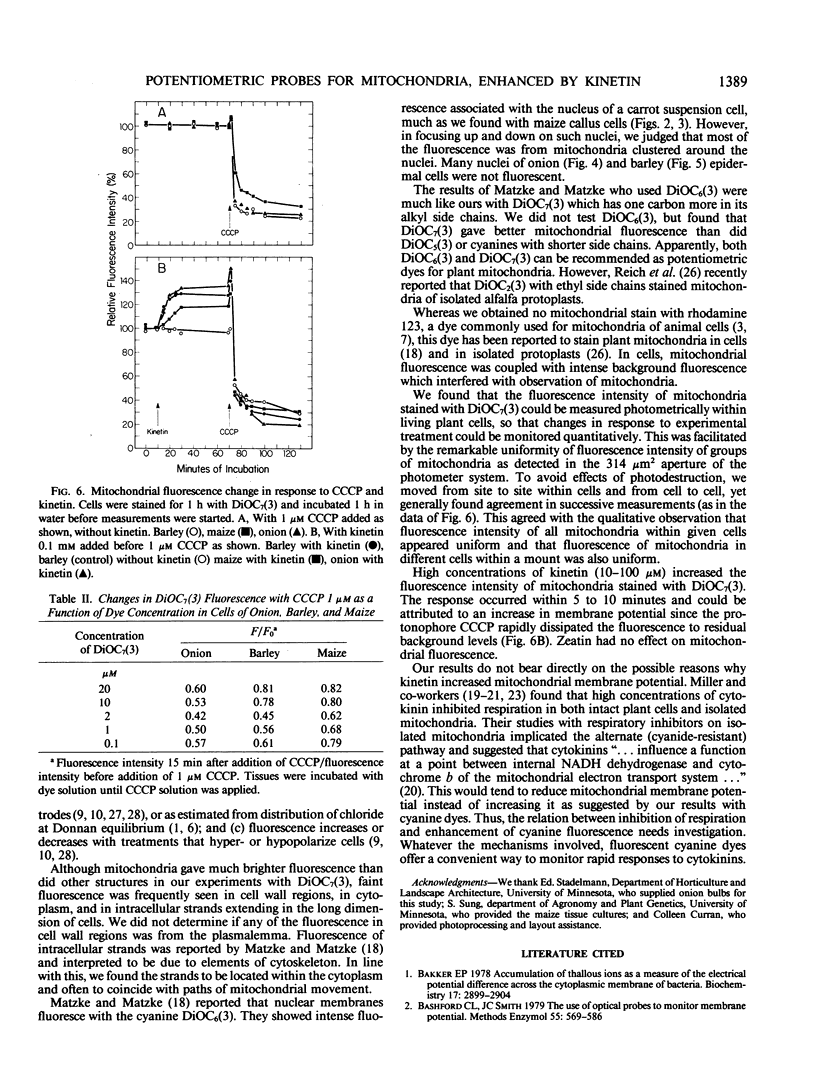
Selected fluorescent dyes were tested for uptake by mitochrondria in intact cells of barley, maize, and onion. The cationic cyanine dye 3,3′-diheptyloxacarbocyanine iodide [DiOC7(3)] accumulated in mitochondria within 15 to 30 minutes without appreciable staining of other protoplasmic constituents. The number, shape, and movement of the fluorescent mitochondria could be seen readily, and the fluorescence intensity of the mitochondria could be monitored with a microscope photometer. Fluorescence was eliminated in 1 to 5 minutes by the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) indicating that maintenance of dye concentration was dependent on the inside-negative transmembrane potential maintained by functional mitochondria. Fluorescence of prestained mitochondria was enhanced within 5 to 10 minutes after addition of 0.1 millimolar kinetin to cells. The fluorescence in kinetintreated cells was dissipated by CCCP. These results suggest that kinetin interacted with respiratory processes resulting in higher potential across the mitochondrial membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P. Accumulation of thallous ions (Tl+) as a measure of the electrical potential difference across the cytoplasmic membrane of bacteria. Biochemistry. 1978 Jul 11;17(14):2899–2904. doi: 10.1021/bi00607a031. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Smith J. C. The use of optical probes to monitor membrane potential. Methods Enzymol. 1979;55:569–586. doi: 10.1016/0076-6879(79)55067-8. [DOI] [PubMed] [Google Scholar]
- Bernal S. D., Shapiro H. M., Chen L. B. Monitoring the effect of anti-cancer drugs on L1210 cells by a mitochondrial probe, rhodamine-123. Int J Cancer. 1982 Aug 15;30(2):219–224. doi: 10.1002/ijc.2910300215. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Salzberg B. M. Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol. 1978;83:35–88. doi: 10.1007/3-540-08907-1_2. [DOI] [PubMed] [Google Scholar]
- Cohen R. L., Muirhead K. A., Gill J. E., Waggoner A. S., Horan P. K. A cyanine dye distinguishes between cycling and non-cycling fibroblasts. Nature. 1981 Apr 16;290(5807):593–595. doi: 10.1038/290593a0. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Staiano-Coico L., Melamed M. R. Increased mitochondrial uptake of rhodamine 123 during lymphocyte stimulation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2383–2387. doi: 10.1073/pnas.78.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J. C., Laris P. C. Electrophysiology of cells and organelles: studies with optical potentiometric indicators. Int Rev Cytol Suppl. 1981;12:177–246. doi: 10.1016/b978-0-12-364373-5.50015-9. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Rink T. J. Potential difference and the distribution of ions across the human red blood cell membrane; a study of the mechanism by which the fluorescent cation, diS-C3-(5) reports membrane potential. J Physiol. 1976 Dec;263(2):287–319. doi: 10.1113/jphysiol.1976.sp011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Laris P. C. Determination of membrane potentials in human and Amphiuma red blood cells by means of fluorescent probe. J Physiol. 1974 Jun;239(3):519–552. doi: 10.1113/jphysiol.1974.sp010581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Rich A. M., Wilkenfeld C., Rutherford L. E., Weissmann G. A carbocyanine dye, DiOC6(3), acts as a mitochondrial probe in human neutrophils. Biochem Biophys Res Commun. 1982 Oct 29;108(4):1495–1501. doi: 10.1016/s0006-291x(82)80076-4. [DOI] [PubMed] [Google Scholar]
- Kovác L., Böhmerová E., Butko P. Ionophores and intact cells. I. Valinomycin and nigericin act preferentially on mitochondria and not on the plasma membrane of Saccharomyces cerevisiae. Biochim Biophys Acta. 1982 Dec 30;721(4):341–348. doi: 10.1016/0167-4889(82)90088-x. [DOI] [PubMed] [Google Scholar]
- Laris P. C., Bahr D. P., Chaffee R. R. Membrane potentials in mitochondrial preparations as measured by means of a cyanine dye. Biochim Biophys Acta. 1975 Mar 20;376(3):415–425. doi: 10.1016/0005-2728(75)90163-2. [DOI] [PubMed] [Google Scholar]
- Miller C. O. Cytokinin inhibition of respiration in mitochondria from six plant species. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4731–4735. doi: 10.1073/pnas.77.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. O. Cytokinin modification of mitochondrial function. Plant Physiol. 1982 Jun;69(6):1274–1277. doi: 10.1104/pp.69.6.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemiosmotic processes. Annu Rev Biochem. 1977;46:996–1005. doi: 10.1146/annurev.bi.46.070177.005024. [DOI] [PubMed] [Google Scholar]
- Moore T. S., Miller C. O. Effects of cytokinins on the respiration of soybean callus tissue. Plant Physiol. 1972 Nov;50(5):594–598. doi: 10.1104/pp.50.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña A., Uribe S., Pardo J. P., Borbolla M. The use of a cyanine dye in measuring membrane potential in yeast. Arch Biochem Biophys. 1984 May 15;231(1):217–225. doi: 10.1016/0003-9861(84)90381-3. [DOI] [PubMed] [Google Scholar]
- Philo R. D., Eddy A. A. The membrane potential of mouse ascites-tumour cells studied with the fluorescent probe 3,3'-dipropyloxadicarbocyanine. Amplitude of the depolarization caused by amino acids. Biochem J. 1978 Sep 15;174(3):801–810. doi: 10.1042/bj1740801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W. N., Salzberg B. M., Cohen L. B., Grinvald A., Davila H. V., Waggoner A. S., Wang C. H. Changes in absorption, fluorescence, dichroism, and Birefringence in stained giant axons: : optical measurement of membrane potential. J Membr Biol. 1977 May 6;33(1-2):141–183. doi: 10.1007/BF01869514. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S. The use of cyanine dyes for the determination of membrane potentials in cells, organelles, and vesicles. Methods Enzymol. 1979;55:689–695. doi: 10.1016/0076-6879(79)55077-0. [DOI] [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]






